
5-Chloro-2-cyanobenzene-1-sulfonyl chloride synthesis
- Product Name:5-Chloro-2-cyanobenzene-1-sulfonyl chloride
- CAS Number:411210-92-9
- Molecular formula:C7H3Cl2NO2S
- Molecular Weight:236.08
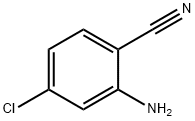
38487-86-4
159 suppliers
$50.00/1g

411210-92-9
20 suppliers
inquiry
Yield:411210-92-9 2.24 g
Reaction Conditions:
Stage #1: 2-amino-4-chlorobenzonitrilewith hydrogenchloride;sodium nitrite in water at 0;
Stage #2: with thionyl chloride;copper(l) chloride in water at 0; for 1 h;Sandmeyer Reaction;
Steps:
29.a a) 5-chloro-2-cyanobenzene-1-sulfonyl Chloride
a)
5-chloro-2-cyanobenzene-1-sulfonyl Chloride
To ice-cooled water (60 mL) was added dropwise thionyl chloride (10 mL) over 1.5 hr and the mixture was stirred at 0° C. for 30 min.
To this solution was added copper(I) chloride (0.13 g) to give solution A.
To the ice-cooled concentrated hydrochloric acid (12 mL) was added 2-amino-4-chlorobenzonitrile (2.0 g) and the mixture was stirred at 0° C. for 10 min.
To the obtained mixture was added dropwise a solution of sodium nitrite (1.10 g) in water (4.4 mL) at 0° C. and the mixture was stirred at 00° C. for 10 min.
The obtained mixture was added dropwise to solution A at 0° C. and the mixture was stirred at 0° C. for 1 hr.
The precipitate was collected by filtration and washed with water to give the title compound as a crude purified product.
The obtained crude purified product was purified by silica gel column chromatography (ethyl acetate/hexane) to give the title compound (2.24 g).
1H NMR (300 MHz, DMSO-d6) δ 7.61 (1H, dd, J=8.2, 2.3 Hz), 7.81 (1H, d, J=2.1 Hz), 7.85 (1H, d, J=8.2 Hz).
References:
US2019/169166,2019,A1 Location in patent:Paragraph 0965; 0966
![6-CHLORO-1,1-DIOXO-1,2-DIHYDRO-1-BENZO[D]ISOTHIAZOL-3-ONE](/StructureFile/ChemBookStructure6/GIF/CB6493556.gif)
46149-10-4
28 suppliers
$45.00/10mg

411210-92-9
20 suppliers
inquiry
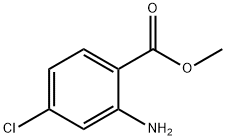
5900-58-3
200 suppliers
$11.00/5g

411210-92-9
20 suppliers
inquiry