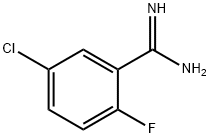
5-chloro-2-fluorobenzamidine synthesis
- Product Name:5-chloro-2-fluorobenzamidine
- CAS Number:674793-32-9
- Molecular formula:C7H6ClFN2
- Molecular Weight:172.59

57381-34-7
187 suppliers
$9.00/5g

674793-32-9
47 suppliers
$45.00/50mg
Yield:674793-32-9 73%
Reaction Conditions:
Stage #1: 5-chloro-2-fluorobenzonitrilewith n-butyllithium;1,1,1,3,3,3-hexamethyl-disilazane in hexanes;diethyl ether at 0 - 20; for 2.08333 h;
Stage #2: with hydrogenchloride;water in hexanes;diethyl ether at 0; for 0.5 h;
Stage #3: with sodium hydroxide in hexanes;diethyl ether;water; pH=14;Product distribution / selectivity;
Steps:
1
Amidine intermediates suitable for preparing certain compounds of formula (I) can be synthesized using lithium bis(trimethylsilyl)amide:To a stirred O0C solution of 1,1,1,3,3,3-Hexamethyldisilazane (63 mL, 0.3 mol) in dry diethyl ether was added dropwise n-Butyl lithium (2M in hexanes, 150 mL, 0.3 mol). A white suspension formed, to which was added 2-Fluoro-5-chlorobenzonitrile (21.0 g, 0.14 mol) over 5 min. The resultant orange mixture was allowed to warm to r.t. and stirred for 2h. The mixture was cooled to O0C and the reaction quenched by the addition of 3M HCl (aq.) (240 mL). The mixture was stirred for 0.5h before water (600 mL) was added. The purple organic layer was discarded and the aqueous layer basified to pH 14 with satd. NaOH (aq.). The aqueous layer was extracted with CHCl3 (5x100 mL) and the organic extracts dried over Na2SO4. Evaporation yielded the desired product as a yellow solid (16.2g, 73% yield).
References:
WO2006/100310,2006,A1 Location in patent:Page/Page column 28

57381-34-7
187 suppliers
$9.00/5g

4039-32-1
410 suppliers
$15.00/10g

674793-32-9
47 suppliers
$45.00/50mg