
5-Chloro-2-hydroxy-3-iodotoluene synthesis
- Product Name:5-Chloro-2-hydroxy-3-iodotoluene
- CAS Number:1257535-34-4
- Molecular formula:C7H6ClIO
- Molecular Weight:268.48

1570-64-5
271 suppliers
$5.00/1g

1257535-34-4
9 suppliers
$45.00/50mg
Yield:1257535-34-4 98%
Reaction Conditions:
with sodium hypochlorite;sodium iodide;sodium hydroxide in methanol;water at 0 - 10; for 2.5 h;
Steps:
1 Step 1: Preparation of 4-chloro-2-iodo-6-methylphenol
To a stirred solution of 5.08 g (35.63 mmol) of 4-chloro-2-methylphenol, 6.42 g (42.83 mmol) of NaI, and 1.74 g (43.50 mmol) of NaOH in 70 mL of methanol at 0-10° C. is added 71 mL (47.69 mmol) of 5% aqueous NaOCl solution (commercial bleach) dropwise over 1.5 hours. After addition of NaOCl solution is complete the reaction mixture is stirred for an additional hour at 0-10° C., then 25 mL of 10 wt. % aqueous sodium thiosulfate is added. The mixture is acidified using 5% HCl, then extracted with methylene chloride (i.e., dichloromethane, DCM). The combined organic phases are washed with an equal volume each of 10 wt. % aqueous sodium thiosulfate, then water, then brine, then dried over anhydrous magnesium sulfate, then filtered through a pad of silica gel, and then concentrated to give crude compound. This crude is recrystallized from hexanes to afford 9.37 g (98%) product as white needles. 1H NMR showed product is 4-chloro-2-iodo-6-methylphenol.
References:
US2014/357918,2014,A1 Location in patent:Paragraph 0084
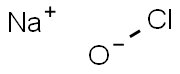
7681-52-9
573 suppliers
$10.00/10ml

1570-64-5
271 suppliers
$5.00/1g

1257535-34-4
9 suppliers
$45.00/50mg