
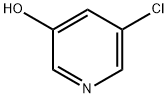
5-chloro-2-nitro-pyridine-3-ol synthesis
- Product Name:5-chloro-2-nitro-pyridine-3-ol
- CAS Number:936247-35-7
- Molecular formula:C5H3ClN2O3
- Molecular Weight:174.54
74115-12-1
205 suppliers
$9.00/1g

936247-35-7
25 suppliers
inquiry
Yield:936247-35-7 82%
Reaction Conditions:
with sulfuric acid;nitric acid at 20;
Steps:
15A 5-Chloro-2-nitropyridin-3-ol
Example 15A
5-Chloro-2-nitropyridin-3-ol
With ice cooling, 30 g of 5-chloropyridin-3-ol (232 mmol, 1 equivalent) were dissolved in 228 ml of concentrated sulphuric acid, and, at 0° C., 24 ml of concentrated nitric acid were added slowly.
The reaction was warmed to RT and stirred overnight.
The mixture was stirred into an ice/water mixture and stirred for another 30 min.
The solid was filtered off, washed with cold water and air-dried.
This gave 33 g (82% of theory) of the title compound, which were used without further purification for the next reaction.
LC-MS (Method 1): Rt=0.60 min
MS (ESneg): m/z=172.9/174.9 (M-H)-
1H NMR (400 MHz, DMSO-d6): δ=7.71 (d, 1H); 8.10 (d, 1H); 12.14 (br. 1H).
References:
US2014/128386,2014,A1 Location in patent:Paragraph 1052; 1053; 1054; 1056