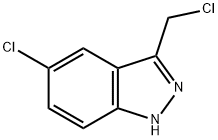
5-Chloro-3-(chloromethyl)-1h-indazole synthesis
- Product Name:5-Chloro-3-(chloromethyl)-1h-indazole
- CAS Number:27328-69-4
- Molecular formula:C8H6Cl2N2
- Molecular Weight:201.05

64605-36-3
2 suppliers
inquiry

27328-69-4
16 suppliers
$94.00/100mg
Yield:27328-69-4 56%
Reaction Conditions:
Stage #1: 5-chloro-2-amino-α-chloroacetophenonewith hydrogenchloride;sodium nitrite in water at 0; for 1 h;
Stage #2: with tin(ll) chloride in water at 0; for 1 h;
Steps:
2; B
To a stirred suspension of 2-amino-5-chloro-?-chloroacetophenone (670 mg, 3,28 mmol) in conc. hydrochloric acid (10 mL) is added a solution of sodium nitrite (250 mg, 3,61 mmol) in water (2 mL) while maintaining the reaction temperature at 0 °C. After 1 h a solution of SnCl2.2H2O (1,78 g, 7,87 mmol) in conc. hydrochloric acid (5 mL) is added to the reaction mixture, which is then stirred at the same temperature for 1 h. Next, ice water is added to the reaction mixture. The precipitate is collected by filtration, washed with water and dried giving crude 5-chloro-3-(chloromethyl)-1H-indazole (370 mg, 1,84 mmol, 56% yield) which is used in the next step without further purification. LC/MS (I) (5-95%, 5 min): 2.67, no mass peak.
References:
EP1674464,2006,A1 Location in patent:Page/Page column 14; 16-17

106-47-8
483 suppliers
$5.00/5G

27328-69-4
16 suppliers
$94.00/100mg