
5-chloro-3H-imidazo[4,5-b]pyridine-2-thiol synthesis
- Product Name:5-chloro-3H-imidazo[4,5-b]pyridine-2-thiol
- CAS Number:40851-97-6
- Molecular formula:C6H4ClN3S
- Molecular Weight:185.63
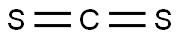
75-15-0
267 suppliers
$40.00/100g
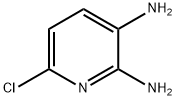
40851-95-4
161 suppliers
$7.00/100mg
![5-chloro-3H-imidazo[4,5-b]pyridine-2-thiol](/CAS/20200401/GIF/40851-97-6.gif)
40851-97-6
14 suppliers
inquiry
Yield:40851-97-6 67%
Reaction Conditions:
Stage #1: carbon disulfide;6-chloropyridine-2,3-diaminewith sodium hydroxide in ethanol;Reflux;
Stage #2: with hydrogenchloride in ethanol;
Steps:
6.2.14. 5-Chloro-1,3-dihydro-2H-imidazo[4,5-b]pyridine-2-thione (9b)
A mixture of 0.144 g (1 mmol) 6-chlorpyridine-2,3-diamine, 20 ml ethanol, one pellet of NaOH and 0.5 ml CS2 is heated to reflux7 as described. When the reaction is completed (TLC control) the mixture is acidified with HCl (2 mol/l), the solvent is evaporated under reduced pressure. The resulting solid is washed several times with H2O. Beige solid, yield: 0.125 g (0.67 mmol), 67%, mp: 290-295 °C. IR: inlMMLBox (cm-1) = 3130; 3063; 2945; 1604. 1H NMR: (200 MHz, DMSO-d6) δ (ppm) = 13.31 (s, 1H, NH); 12.86 (s, 1H, NH); 7.50 (d, 1H, J = 8.2 Hz, H arom.); 7.19 (d, 1H, J = 8.2 Hz, H arom.). 13C NMR: (50 MHz, DMSO-d6) δ (ppm) = 170.36; 145.80; 142.48; 124.73; 118.75; 117.43.
References:
Freitag, Marcus;Schemies, Joerg;Larsen, Tim;El Gaghlab, Khattab;Schulz, Felix;Rumpf, Tobias;Jung, Manfred;Link, Andreas [Bioorganic and Medicinal Chemistry,2011,vol. 19,# 12,p. 3669 - 3677] Location in patent:experimental part