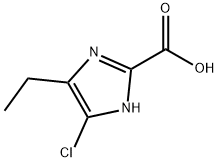
5-chloro-4-ethyl-1H-iMidazole-2-carboxylic acid synthesis
- Product Name:5-chloro-4-ethyl-1H-iMidazole-2-carboxylic acid
- CAS Number:1171124-67-6
- Molecular formula:C6H7ClN2O2
- Molecular Weight:174.59
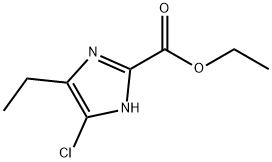
1171124-66-5
3 suppliers
inquiry

1171124-67-6
4 suppliers
inquiry
Yield:1171124-67-6 100%
Reaction Conditions:
Stage #1: ethyl 4-chloro-5-ethyl-1H-imidazole-2-carboxylatewith water;lithium hydroxide in methanol;dichloromethane at 70;
Stage #2: with hydrogenchloride in water; pH=7;
Steps:
1.1d
(1d) 4-Chloro-5-ethyl-1H-imidazole-2-carboxylic acid Ethyl 4-chloro-5-ethyl-1H-imidazole-2-carboxylate obtained in Example (1c) (0.52 g, 2.56 mmol) was dissolved in methanol (8 mL) and dichloromethane (2 mL), and the solution was slowly added dropwise to a 3 N aqueous lithium hydroxide solution (3.5 mL, 10.5 mmol) heated to 70°C. Following stirring at that temperature for 40 minutes, the reaction solution was concentrated under reduced pressure and 1 N hydrochloric acid was added dropwise until the pH was 7. Thereafter, purification by reverse phase silica gel column chromatography (elution solvent: distilled water) gave 0.48 g of the title compound as a white solid (100%). 1H NMR spectrum (400 MHz, DMSO-d6) δ ppm: 1.10 (3H, t, J = 7.32 Hz), 2.44-2.51 (2H, m).
References:
EP2226322,2010,A1 Location in patent:Page/Page column 28