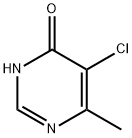
5-CHLORO-6-METHYLPYRIMIDIN-4(1H)-ONE synthesis
- Product Name:5-CHLORO-6-METHYLPYRIMIDIN-4(1H)-ONE
- CAS Number:7752-72-9
- Molecular formula:C5H5ClN2O
- Molecular Weight:144.56
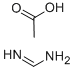
3473-63-0
482 suppliers
$5.00/25g
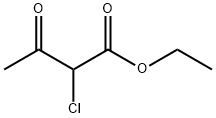
609-15-4
442 suppliers
$6.00/25g

7752-72-9
53 suppliers
$40.00/100mg
Yield:7752-72-9 66%
Reaction Conditions:
Stage #1: formamidine acetic acidwith sodium methylate in methanol at 20; for 2 h;
Stage #2: ethyl 2-chloro-3-oxo-butyrate in methanol at 20;
Steps:
1.1 preparation of 4-hydroxyl-5-chloro-6-methylpyrimidine
The preparation of 4-hydroxyl-5-chloro-6-methylpyrimidine ;8.80g (0.16mol) of CH3ONa in methanol was added slowly to a solution of 11.30g (0.11mol of formimidamide in 50 mL of methanol at room temperature under stirring, the mixture was stirred for another 2 hrs after addition at room temperature. ;Followed by addition of 11.17g ( 0.068mol ) of ethyl 2-chloro-3-oxobutanoate, the mixture was continued stirring for another 5-7 hrs at room temperature. ;After the reaction was over by Thin-Layer Chromatography monitoring, the reaction mixture was concentrated under reduced pressure and pH was adjusted to 5-6 with HCl, and then filtered to afford orange-yellow soilid, the water phase was extracted with ethyl acetate (3x50mL), dried over anhydrous magnesium sulfate, filtered and then concentrated under reduced pressure. ;The residue was dissolved to 50ml of ethyl acetate, stand overnight to obtain 6.48g as orange-yellow soilid with yield of 66%. m.p. 181~184°C.
References:
EP2913325,2015,A1 Location in patent:Paragraph 0451; 0452
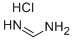
6313-33-3
190 suppliers
$9.00/1g

609-15-4
442 suppliers
$6.00/25g

7752-72-9
53 suppliers
$40.00/100mg
