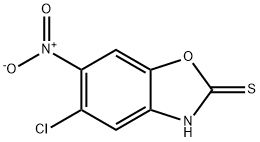
5-chloro-6-nitro-1,3-benzoxazole-2-thiol synthesis
- Product Name:5-chloro-6-nitro-1,3-benzoxazole-2-thiol
- CAS Number:199293-10-2
- Molecular formula:C7H3ClN2O3S
- Molecular Weight:230.63
Yield:-
Reaction Conditions:
with potassium hydroxide in ethanol at 70 - 80; for 17 h;
Steps:
1.1 Step-1 : 5-chloro-6-nitrobenzo[d]oxazole-2-thiol.
Step-1 : 5-chloro-6-nitrobenzo[d]oxazole-2-thiol. To a solution of 2-amino-4-chloro-5-nitrophenol (2g, 10.781 mmol) in ethanol (30 ml), was added potassium hydroxide (0.75g, 12.938 mmol) followed Carbon disulfide (20ml). The reaction mixture was stirred at 70-80°C for 17h. The progress of the reaction was monitored by TLC and then it was cooled to 20-30°C, after concentrating the mixture under reduced pressure, it was extracted with water (30 ml) and dichloromethane (2x30 ml). The organic layer was collected, washed with brine, dried over sodium sulfate and concentrated under reduced pressure to get the crude product(2.3g,92%). ? NMR (400 MHz, DMSO-£/6): δ (ppm)7.88 (s, 1H), 7.24(s, 1H). MS (ES) m/e 231 (M+H) +.
References:
WO2013/42137,2013,A1 Location in patent:Page/Page column 32

