
5-(CHLOROMETHYL)-3-ETHYL-1,2,4-OXADIAZOLE synthesis
- Product Name:5-(CHLOROMETHYL)-3-ETHYL-1,2,4-OXADIAZOLE
- CAS Number:50737-34-3
- Molecular formula:C5H7ClN2O
- Molecular Weight:146.57
29335-36-2
83 suppliers
$29.00/100mg

79-04-9
375 suppliers
$12.00/5g

50737-34-3
19 suppliers
$65.00/50mg
Yield:50737-34-3 14%
Reaction Conditions:
for 1.16667 h;Heating / reflux;
Steps:
B.62.1
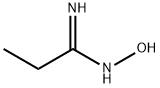
To crude N-hydroxypropanimidamide (80 g, 0.91 mol), obtained from propionitrile and hydroxylamine (Moloney, G. P.; Martin, G. R.; Mathews, N.; Maclennan, S.; Dodsworth, S.; Sang, P. Y.; Knight, C.; Maxwell, M.; Glen, R. C. J. Chem. Soc Perkin I 1999, 19, 2725), was added chloroacetyl chloride (411 g, 3.64 mol, 4 equiv). After the initial exothermic reaction subsided, the mixture was refluxed for 70 min. The reaction mixture was cooled to room temperature, and the excess chloroacetyl chloride evaporated. The residue was dissolved in ethyl acetate, diluted with hexanes, and filtered to remove dark solid impurities. The filtrate was evaporated, treated with ice-cold aqueous NaHCO3, and extracted with ethyl acetate (2×150 mL). The extract was dried over Na2SO4, filtered, and the solvent evaporated, affording 76.25 g of an oil. This was subjected to fractional distillation, affording 16.5 g (14%) of the title product (bp 36° C./0.05 Torr; lit bp 88° C./35 Torr: Hagerty, J. D. U.S. Pat. No. 3,956,498, May 11, 1976). 1H NMR (CDCl3) d 1.31 (t, J=7 Hz, 3H), 2.75 (q, J=7 Hz, 2H), 4.64 (s, 2H). LC-MS (APCI) calcd for C5H7ClN2O: 146.02; found (M+H+): 147.0 m/z.
References:
US2005/176701,2005,A1 Location in patent:Page/Page column 150-151