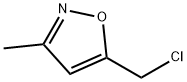
5-(CHLOROMETHYL)-3-METHYLISOXAZOLE synthesis
- Product Name:5-(CHLOROMETHYL)-3-METHYLISOXAZOLE
- CAS Number:40340-41-8
- Molecular formula:C5H6ClNO
- Molecular Weight:131.56
Yield:40340-41-8 327 mg
Reaction Conditions:
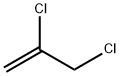
with triethylamine at 20; for 1 h;Reagent/catalyst;
Steps:
5-(Chloromethyl)-3-methylisoxazole (5).
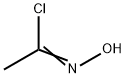
A drop of pyridine was added and then a solution of acetaldoxime(237 mg, 4 mmol) in DCP (2 ml) was added dropwise to asuspension of NCS (560 mg, 4.2 mmol) in DCP (5 ml). Aslight heating, dissolution, and precipitation were observed.After 3 h, a solution of Et3N (910 mg, 9 mmol) in DCP(2 ml) was added with vigorous stirring, resulting inheating and formation of a large volume of precipitate.Stirring was continued at room temperature for 1 h, thereaction mixture was diluted with pentane (20 ml), theprecipitate was filtered off, and the filtrate was evaporated.The residue was purified by flash chromatography on silicagel, eluent CH2Cl2. Yield 327 mg (62%, method I), 42 mg(8%, method II), colorless oil. 1H NMR spectrum, δ, ppm:2.27 (3, s, CH3); 4.55 (2, s, 2Cl); 6.14 (1, s, -4).13C NMR spectrum, δ, ppm: 11.4; 34.5; 104.4; 160.1;167.2. Analytical data match those published previously.5d
References:
Kondrashov, Evgeniy V.;Shatokhina, Nina S. [Chemistry of Heterocyclic Compounds,2019,vol. 55,# 12,p. 1228 - 1232][Khim. Geterotsikl. Soedin.,2019,vol. 55,# 12,p. 1228 - 1232,5]
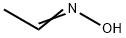
107-29-9
217 suppliers
$9.00/1g
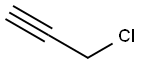
624-65-7
386 suppliers
$26.00/5mL

40340-41-8
30 suppliers
$50.00/25mg
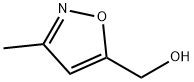
14716-89-3
73 suppliers
$45.00/50mg

40340-41-8
30 suppliers
$50.00/25mg
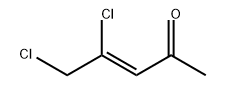
54813-96-6
0 suppliers
inquiry

40340-41-8
30 suppliers
$50.00/25mg
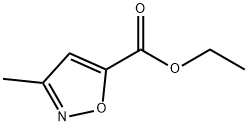
63366-79-0
82 suppliers
$45.00/50mg

40340-41-8
30 suppliers
$50.00/25mg