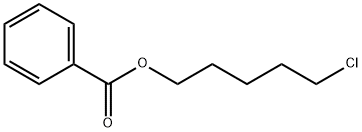
5-Chloropentyl benzoate synthesis
- Product Name:5-Chloropentyl benzoate
- CAS Number:55092-47-2
- Molecular formula:C12H15ClO2
- Molecular Weight:226.7
142-68-7
184 suppliers
$14.00/25mL

98-88-4
575 suppliers
$10.00/5g

55092-47-2
22 suppliers
$28.60/250MG
Yield:55092-47-2 92.6%
Reaction Conditions:
with palladium diacetate at 100; for 2 h;Microwave irradiation;
Steps:
5-Chloropentyl benzoate (11a)
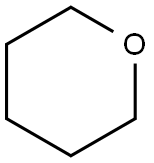
Benzoyl chloride (1 g, 7.11 mmol) was mixed with 7.11 mL of tetrahydropyran to make a 1M solution, to which 2 mol% Pd(OAc)2 (31.9 mg, 0.142 mmol) was added. The mixture was stirred at room temperature for 24 h (since 4 h produced only traces of the product), and the excess of tetrahydropyran was removed by rota-evaporation, under a reduced pressure. The residue was purified on a CombiFlash using a gradient of hexane - ethyl acetate, with the polarity ranging from pure hexane to a mixture of hexane-ethyl acetate (95:5). The compound came out in 8.5 minutes as clear oily material (366 mg, 22.7%) after the removal of the solvent under a reduced pressure. However, when the reaction mixture is heated at 100 oC under a microwave irradiation for 2 h, the yield of the reaction increases drastically to 92.6% (1.49 g of product). 1H NMR (400 MHz, CDCl3): δ (ppm) 1.61 (2H, m), 1.80 (4H, m), 3.54 (2H, t, J = 6.4 Hz), 4.32 (2H, t, J = 6.4 Hz), 7.42 (2H, dd, J = 8.0 and 7.6 Hz), 7.52 (1H, t, J = 7.2 Hz), 8.04 (2H, d, J = 8.0 Hz). 13C NMR (100 MHz, CDCl3): δ (ppm) 23.5, 28.0, 32.2, 44.9, 64.8, 128.4, 129.5, 130.4, 132.9, 166.6. HRMS (ESI/Q-TOF): [M + H]+ Calcd for C12H16ClO2 227.0833; Found 227.0837.
References:
Fotie, Jean;Adolph, Brandy R.;Bhatt, Shreya V.;Grimm, Casey C. [Tetrahedron Letters,2017,vol. 58,# 49,p. 4648 - 4651] Location in patent:supporting information

628-76-2
182 suppliers
$24.00/25mL
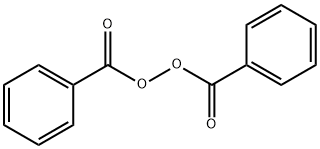
94-36-0
481 suppliers
$22.00/25g

55092-47-2
22 suppliers
$28.60/250MG

142-68-7
184 suppliers
$14.00/25mL

98-88-4
575 suppliers
$10.00/5g

55092-47-2
22 suppliers
$28.60/250MG
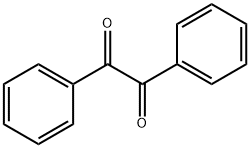
134-81-6
464 suppliers
$9.00/25g

98-88-4
575 suppliers
$10.00/5g

55092-47-2
22 suppliers
$28.60/250MG