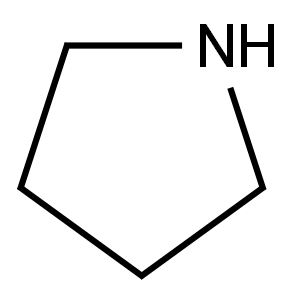
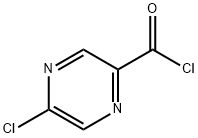
(5-Chloropyrazin-2-yl)(pyrrolidin-1-yl)Methanone synthesis
- Product Name:(5-Chloropyrazin-2-yl)(pyrrolidin-1-yl)Methanone
- CAS Number:1245215-68-2
- Molecular formula:C9H10ClN3O
- Molecular Weight:211.65
Yield:1245215-68-2 84.5%
Reaction Conditions:
with triethylamine in dichloromethane at 0 - 20;
Steps:
Preparation of (5-chloropyrazin-2-yl)(pyrrolidin-1-yl)methanone (SM-1):(SM-1 ) δ-chloropyrazine^-carboxylic acid (1 .00 gram, 6.31 mmol) in dichloromethane (30 ml_) was treated with catalytic amount of dimethylformamide, followed by (COCI)2 (0.85 ml_, 9.46 mmol). The resulting mixture was stirred over night. The reaction was concentrated and dried under vacuum to give desired δ-chloropyrazine^-carbonyl chloride as a solid (1 .05 g, 100%). δ-chloropyrazine^-carbonyl chloride (670mg, 3.79mmol) was dissolved in dichloromethane (1 OmL) and cooled to O0C. Thethylamine (1 .58ml_, 1 1 .4mmol) and pyrrolidine (0.32ml_, 3.79mmol) were then added successively drop-wise. Following the addition, the ice bath was removed and the reaction was allowed to warm to room temperature and stir for 1 hour. The reaction was then diluted with dichloromethane and washed with 1 N HCI, water, and brine. The organics were dried over sodium sulfate, filtered, and concentrated. Purification by column chromatography eluting with 30 - 80% ethyl acetate in hexane afforded the desired (5-chloropyrazin- 2-yl)(pyrrolidin-1 -yl)methanone (SM-1 : 677.0mg, 84.5%). 1H NMR (400 MHz, CHLOROFORM-d) δ ppm 1 .86 - 1 .99 (m, 4 H),3.65 - 3.71 (m, 2 H), 3.73 - 3.79 (m, 2 H), 8.52 (d, J=1 .37 Hz, 1 H), 8.93 (d, J=1 .37 Hz, 1 H).
References:
WO2010/103438,2010,A1 Location in patent:Page/Page column 36