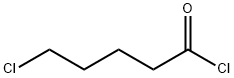
5-Chlorovaleryl chloride synthesis
- Product Name:5-Chlorovaleryl chloride
- CAS Number:1575-61-7
- Molecular formula:C5H8Cl2O
- Molecular Weight:155.02
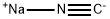
773837-37-9
0 suppliers
inquiry

110-56-5
297 suppliers
$15.00/25mL

1575-61-7
346 suppliers
$14.00/1g
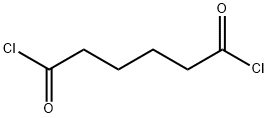
111-50-2
212 suppliers
$10.00/1g
Yield:1575-61-7 422 kg ,111-50-2 163 kg
Reaction Conditions:
Stage #1: sodium cyanide;1,4-dichlorobutanewith tetrabutylammomium bromide in water at 80 - 85; for 6 h;Large scale;
Stage #2: with hydrogenchloride in water at 55 - 70; for 5 h;Large scale;
Stage #3: with thionyl chloride at 20 - 30; for 18 h;Large scale;
Steps:
3
1270Kg of 1,4-dichlorobutane and 16.5Kg of tetrabutylammonium bromide catalyst were put into a 3000L reactor.Warm to 80-85 ° C with stirring.1060 Kg of a 30% aqueous solution of sodium cyanide was uniformly added dropwise over 5 hours.After the addition was completed, the reaction was further carried out at 80-85 ° C for 1 hour.Cool the kettle liquid to 25 ° C,Use a vacuum pump or transfer pump to drive into the high 3000L phase separation tank.The lower organic phase is a solution of 5-chlorovaleronitrile and adiponitrile in 1,4-dichlorobutane.The organic phase was separated and returned to the kettle.The upper aqueous phase is discharged into the wastewater system for treatment.Adding 30% industrial hydrochloric acid to 1740 Kg in the reaction kettle.The mixture was heated to 55-70 ° C with stirring for 5 hours. After the reaction, cool to 45 ° C,Add 110Kg of water to dissolve the solid ammonium chloride completely.The lower organic phase was statically separated into a solution of 5-chloropentanoic acid and adipic acid in 1,4-dichlorobutane.The organic phase is separated and returned to the reaction vessel.Add 1700 Kg of thionyl chloride to the reaction kettle.The reaction was carried out at 20-30 ° C for 18 hours.The reaction liquid was transferred to a 2000 L vacuum distillation column in a column reactor.Excessive amount of thionyl chloride is distilled off under normal pressure.Then increase the vacuum to 20mmHg,Distilling 1,4-dichlorobutane can be used in the next batch of cyanidation reaction,The vacuum is increased to 2 mmHg, and 422 kg of 5-chlorovaleryl chloride is distilled off.Adipoyl chloride 163Kg.
References:
CN107827740,2018,A Location in patent:Paragraph 0017; 0018; 0019; 0020; 0021; 0022; 0023-0028
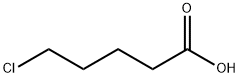
1119-46-6
225 suppliers
$8.00/5g

1575-61-7
346 suppliers
$14.00/1g
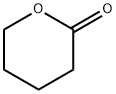
542-28-9
313 suppliers
$5.00/5g
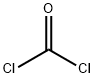
75-44-5
2 suppliers
inquiry
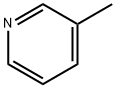
108-99-6
320 suppliers
$9.00/5g

1575-61-7
346 suppliers
$14.00/1g

542-28-9
313 suppliers
$5.00/5g

75-44-5
2 suppliers
inquiry
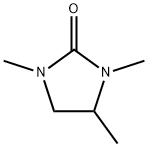
24044-24-4
3 suppliers
inquiry

1575-61-7
346 suppliers
$14.00/1g

542-28-9
313 suppliers
$5.00/5g

75-44-5
2 suppliers
inquiry
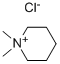
24307-26-4
242 suppliers
$9.00/250mg

1575-61-7
346 suppliers
$14.00/1g