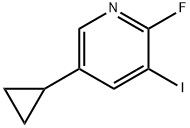
5-Cyclopropyl-2-fluoro-3-iodopyridine synthesis
- Product Name:5-Cyclopropyl-2-fluoro-3-iodopyridine
- CAS Number:1034467-82-7
- Molecular formula:C8H7FIN
- Molecular Weight:263.05
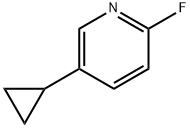
1034467-80-5
40 suppliers
$45.00/10mg

1034467-82-7
7 suppliers
inquiry
Yield: 68%
Reaction Conditions:
Stage #1:5-cyclopropyl-2-fluoropyridine with lithium diisopropyl amide in tetrahydrofuran at -75; for 3.5 h;
Stage #2: with iodine in tetrahydrofuran for 2 h;
Steps:
47
Preparation 47; S-Cyclopropyl^-fluoro-S-iodo-pyridine; Cool a solution of 5-cyclopropyl-2-fluoro-pyridine (1.3 g, 9.5 mmol) in THF (20 mL) to -75 0C in a dry ice-acetone bath under nitrogen. Add lithium diisopropylamide (2 M in THF, 6 mL, 12 mmol) over a period of 30 min. Stir the mixture for an additional 3 hours. Add iodine (2.9 g, 11.4 mmol, dissolved in 50 mL of THF) and stir the mixture for 2 hours. Add water (100 mL) and allow the temperature to rise to room temperature over 1 hour while stirring. Treat the mixture with saturated aqueous sodium thiosulfate solution (50 mL). Extract the solution with ether. Concentrate the solution in vacuo to brown oil. Purify by column chromatography (hexane to 20 % ethyl acetate in hexane) to afford the title compound (1.7 g, 68 %) as a yellow oil. 1H NMR (40OMHz-CDCl3) δ 8.03 (dd, J= 3, 8 Hz, IH), 7.99 (s, IH), 0.91-1.00 (m, 2H), 0.71-0.78 (m, 2H).
References:
ELI LILLY AND COMPANY WO2008/76705, 2008, A1 Location in patent:Page/Page column 20
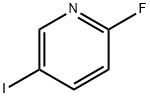
171197-80-1
282 suppliers
$20.00/1g

1034467-82-7
7 suppliers
inquiry