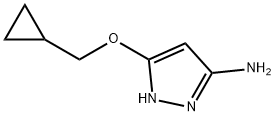
5-(cyclopropylMethoxy)-1H-pyrazol-3-aMine synthesis
- Product Name:5-(cyclopropylMethoxy)-1H-pyrazol-3-aMine
- CAS Number:852443-66-4
- Molecular formula:C7H11N3O
- Molecular Weight:153.18
2516-33-8
289 suppliers
$9.00/1g

53666-79-8
50 suppliers
inquiry

852443-66-4
33 suppliers
$77.00/100mg
Yield:852443-66-4 15%
Reaction Conditions:
with di-isopropyl azodicarboxylate;triphenylphosphine in tetrahydrofuran;DMF (N,N-dimethyl-formamide) at 25;
Steps:
44
Triphenylphosphine (16.0 g, 61 mmol),5-amino-2H-pyrazol-3-ol (5.5 g, 56 mmol), and cyclopropyl methanol (4.4 g, 61 mmol) were dissolved in THF (100 ml), to which was slowly added the diisopropyl azodicarboxylate (12 ml, 61 mmol) solution in THF (50 ml). The reaction mixture was stirred for 1 hour, diluted with DMF (45 ml), and allowed at25 C overnight. The solvent was removed under reduced pressure. The resulted residue was treated with water, extracted with EtOAc twice and DCM once. The combined organics were dried over anhydrous sodium sulfate, concentrated under reduced pressure, and purified by column chromatography (EtOAc) to give the title compound (1.3 g, 15%). MS: Calcd.: 153; Found: [M+H] + 154.
References:
WO2005/49033,2005,A1 Location in patent:Page/Page column 103