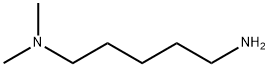
5-(Dimethylamino)pentylamine synthesis
- Product Name:5-(Dimethylamino)pentylamine
- CAS Number:3209-46-9
- Molecular formula:C7H18N2
- Molecular Weight:130.23
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

3209-46-9
55 suppliers
$74.00/1g
Yield: 42%
Reaction Conditions:
with hydrazine hydrate in ethanol;water for 12 h;Reflux;Gabriel Amine Synthesis;
Steps:
1.1.3. General procedure for preparation of compounds 18 to 22
General procedure: Compound 13-17 (1 eq.) was dissolved in EtOH (100 mL for 2.5 to 5 g of phthalimide) and hydrazine hydrate (aqueous solution, 60% wt, 4 eq.) was added dropwise under stirring. The mixture was refluxed for 12 h and then cooled down to rt. The obtained suspension was filtered and the precipitate was washed thoroughly with EtOH. The filtrate was cooled in an ice-water bath and treated dropwise with a concentrated aqueous HCl solution (6 eq.). The formed solid was removed by fitration and washed with EtOH (approximately 100 mL for 5 g of phthalimide). One supplementary fraction of solid was removed by reacidification of the filtrate (if precipitate didn’t appear, the mixture was allowed to cool at 4 °C for 1 h). The filtrate was concentrated under reduced pressure, cooled in an ice-water bath and then gently dissolved in a minimum amount of an aqueous KOH solution (50% wt). The obtained solution was extracted with Et2O (3 × 75 mL for 5 g of phthalimide). The combined organic layers were dried over MgSO4, filtered and concentrated under reduced pressure to afford a transparent to pale yellow oily liquid. This crude product was used directly for the next step or purified by distillation under reduced pressure to yield pure amines. N,N-dimethylpentane-1,5-diamine (18a) Deprotection according to the general procedure starting from compound 13a (9.14 g, 35.1 mmol) followed by distillation under reduced pressure afforded pure amine 18a (1.92 g, 14.7 mmol). Yield 42%; bp 85 °C at 28 Torr (Lit.2 88-89 °C at 30 Torr); IR (ATR) ν cm-1 3356, 3278, 2930, 2856, 2814, 2762, 1593, 1459, 1382, 1040, 844; 1H NMR (CDCl3, 400 MHz) δ 2.64 (t, 2H, 3J = 7.0 Hz, NH2CH2), 2.22-2.17 (m, 2H, CH2N(CH3)2), 2.15 (s, 6H, N(CH3)2), 1.67 (br.s, 2H, NH2), 1.46-1.37 (m, 4H, NH2CH2CH2CH2CH2), 1.32-1.25 (m, 2H, NH2CH2CH2CH2); 13C NMR (CDCl3, 75 MHz) δ 59.80 (CH2N(CH3)2), 45.39 (2C, N(CH3)2), 42.11 (NH2CH2), 33.71 (NH2CH2CH2), 27.53 (CH2CH2N(CH3)2), 24.75 (NH2CH2CH2CH2).
References:
Ghedira, Donia;Voissière, Aurélien;Peyrode, Caroline;Kraiem, Jamil;Gerard, Yvain;Maubert, Elise;Vivier, Magali;Miot-Noirault, Elisabeth;Chezal, Jean-Michel;Farhat, Farhat;Weber, Valérie [European Journal of Medicinal Chemistry,2018,vol. 158,p. 51 - 67] Location in patent:supporting information
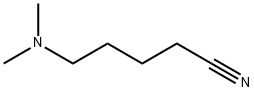
3209-45-8
14 suppliers
inquiry

3209-46-9
55 suppliers
$74.00/1g
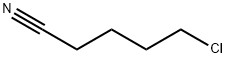
6280-87-1
213 suppliers
$10.00/1g

3209-46-9
55 suppliers
$74.00/1g

67-56-1
739 suppliers
$7.29/5ml-f

462-94-2
207 suppliers
$24.00/1g

32752-52-6
2 suppliers
inquiry

56992-95-1
0 suppliers
inquiry

3209-46-9
55 suppliers
$74.00/1g