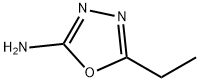
5-ETHYL-1,3,4-OXADIAZOL-2-YLAMINE synthesis
- Product Name:5-ETHYL-1,3,4-OXADIAZOL-2-YLAMINE
- CAS Number:3775-61-9
- Molecular formula:C4H7N3O
- Molecular Weight:113.12
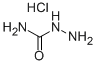
563-41-7
533 suppliers
$5.00/25g
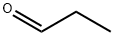
123-38-6
432 suppliers
$14.00/5mL

3775-61-9
45 suppliers
$215.00/1g
Yield:3775-61-9 41%
Reaction Conditions:
Stage #1: hydrazinecarboxamide monohydrochloride;propanalwith anhydrous Sodium acetate in tetrahydrofuran;lithium hydroxide monohydrate; for 1.5 h;Cooling with ice;
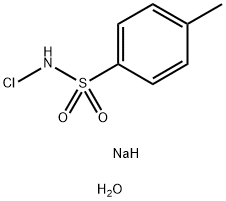
Stage #2: with chloramine-T;potassium carbonate in tetrahydrofuran;lithium hydroxide monohydrate at 20; for 3 h;Reflux;
Steps:
C8 Example C8
Example C8
Production of 5-ethyl-1,3,4-oxadiazol-2-amine
A solution of semicarbazide hydrochloride (1.22 g, 10.9 mmol) and sodium acetate (0.897 g, 10.9 mmol) in water (10 mL) was cooled in ice, then propionaldehyde (0.605 g, 10.4 mmol) and tetrahydrofuran (THF) (3.0 mL) were added thereto, and the mixture was stirred. 1.5 hours later, tetrahydrofuran (THF) (27 mL), potassium carbonate (3.60 g, 26.0 mmol), and chloramine T trihydrate (4.84 g, 17.2 mmol) were added to the reaction solution, and the mixture was stirred at room temperature for 1 hour and then heated to reflux. 2 hours later, the reaction solution was cooled to room temperature and washed with a 2:1 mixed solution of a 20% sodium bisulfite aqueous solution and 30% aqueous sodium chloride solution.
Toluene was added to the obtained organic layer, followed by extraction with a 4:1 mixed solution of 2 mol/L hydrochloric acid and 30% aqueous sodium chloride solution.
The obtained aqueous layer was adjusted to pH 12 or higher by the addition of a 10 mol/L sodium hydroxide aqueous solution, and this solution was then concentrated under reduced pressure.
The obtained residue was purified by silica gel column chromatography (chloroform/methanol) to obtain the title compound (0.485 g, yield: 41%) as a white solid.
1H-NMR (400 MHz, DMSO-D6) δ: 6.81 (2H, brs), 2.63 (2H, q, J=7.7 Hz), 1.17 (3H, t, J=7.7 Hz)
ESI-MS (m/z): 114 (M+H)+
References:
US2022/169640,2022,A1 Location in patent:Paragraph 0281-0283

563-41-7
533 suppliers
$5.00/25g
7080-50-4
280 suppliers
$7.00/25g
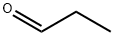
123-38-6
432 suppliers
$14.00/5mL

3775-61-9
45 suppliers
$215.00/1g

628-07-9
0 suppliers
inquiry

3775-61-9
45 suppliers
$215.00/1g