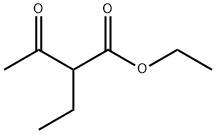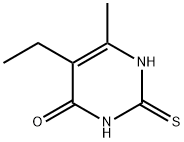
5-Ethyl-2-mercapto-6-methylpyrimidin-4(3H)-one synthesis
- Product Name:5-Ethyl-2-mercapto-6-methylpyrimidin-4(3H)-one
- CAS Number:39083-15-3
- Molecular formula:C7H10N2OS
- Molecular Weight:170.23
Yield:39083-15-3 82%
Reaction Conditions:
with potassium hydroxide in ethanol; for 5 h;Reflux;
Steps:
Step 1
Ethyl 2-ethyl-3-oxobutanoate (1.6 mL, 10 mmol) was added to a stirred suspension of thiourea (766 mg, 10 mmol) and KOH (677 mg, 12 mmol) in EtOH (20 mL). The solution was refluxed for 5 h and completion of the reaction was confirmed by LCMS. The solid formed was collected by filtration and then dissolved in H2O. The solution was then acidified with IN HC1 to pH 1 to give white precipitate of 5-ethyl-6-methyl-2-thioxo-2,3-dihydropyrimidin-4(lH)-one that was filtered and dried under vacuum (1.39 g, 82% yield). 1H NMR (400 MHz, DMSO-rL) d 12.29 (s, 1H), 12.06 (s, 1H), 2.24 (q, J = 8.0 Hz, 2H), 2.11 (s, 3H), 0.92 (t, J= 8.0 Hz, 3H). ESI-MS (m/z): 171.1 [M+H]+.
References:
WO2021/77102,2021,A1 Location in patent:Page/Page column 60-61
51756-08-2
51 suppliers
$50.89/25g

17356-08-0
3 suppliers
inquiry

39083-15-3
17 suppliers
$168.30/5MG