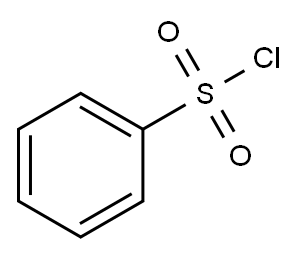
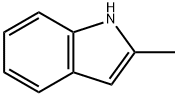
5-fluoro-1-phenylsulfonyl-1H-indole synthesis
- Product Name:5-fluoro-1-phenylsulfonyl-1H-indole
- CAS Number:99532-44-2
- Molecular formula:C14H10FNO2S
- Molecular Weight:275.3
Yield:99532-44-2 100%
Reaction Conditions:
Stage #1: 5-fluoro-1H-indolewith n-butyllithium in tetrahydrofuran;hexane at -78 - 0; for 1 h;
Stage #2: benzenesulfonyl chloride in tetrahydrofuran;hexane at 0 - 20; for 2.5 h;
Steps:
82
Example 82: Intermediate 67--5-FLUORO-1-(PHENYLSULFONYL)-LH-INDOLE [0469] To 5-fluoroindole (0.5g, 3.7mmol) in anhydrous tetrahydrofuran (8.5 ml), under nitrogen at -78°C, was added dropwise 2.5 M nBuLi/hexane (1.6 ml, 4.1 mmol) and the reaction mixture stirred for 40 min at-78 C. It was transferred to an ice bath and stirred for another 20MIN. BENZENESULFONYL chloride (0.5 ml, 3. 88 mmol) was then added dropwise and the reaction mixture slowly brought to room temperature, and stirred for 2.5 hrs. It was poured over 2% NAHC03 and extracted with diethyl ether. The organic extracts were washed with 2% NAHCO3, water and brine, dried over anhydrous magnesium sulfate, filtered and concentrated under vacuum. Chromatography ( (9 : 1) Hexane-EtOAc) afforded 1.05 g (100%) of the title compound as A white solid. The product was characterized BY 1HNMR.
References:
WO2005/12291,2005,A1 Location in patent:Page/Page column 86-87

352-70-5
251 suppliers
$14.00/25mL

99532-44-2
8 suppliers
inquiry
446-33-3
250 suppliers
$20.00/25g

99532-44-2
8 suppliers
inquiry

32989-58-5
0 suppliers
inquiry

99532-44-2
8 suppliers
inquiry