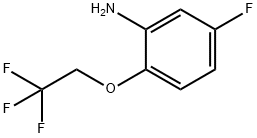
5-FLUORO-2-(2,2,2-TRIFLUOROETHOXY)ANILINE synthesis
- Product Name:5-FLUORO-2-(2,2,2-TRIFLUOROETHOXY)ANILINE
- CAS Number:334929-99-6
- Molecular formula:C8H7F4NO
- Molecular Weight:209.14
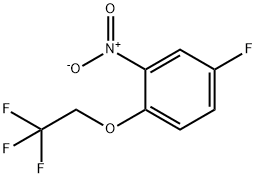
334929-98-5
7 suppliers
inquiry

334929-99-6
18 suppliers
$110.00/50mg
Yield:334929-99-6 94%
Reaction Conditions:
with hydrogen;palladium 10% on activated carbon in methanol; under 2585.81 - 3102.97 Torr;
Steps:
f
Trifluoroethanol (1 equiv.) was taken in dichloromethane, cooled to about 5-100C and stirred for about 15 minutes. Triethylamine was added to this mixture and again stirred for about 10 minutes. Methanesulphonylchloride (1.5 equiv.) in dichloromethane was the added dropwise to the solvent mixture. The reaction mixture was then allowed to come to room temperature and stirred overnight at this temperature. The reaction mixture was poured into 5% sodium bicarbonate solution and stirred for about 15 minutes. The dichloromethane layer was separated and the aqueous layer was extracted with dichloromethane. The organic extract was dried, concentrated to afford the product in 90 % yield. To a suspension of sodium hydride (1 equiv.) in hexamethylphosphoric triamide, cooled to about 00C, 4-fluoro-2-nitro phenol was added portionwise and the reaction mixture was stirred for about 30 minutes. The above product (1.2 equiv.) was then added portionwise, the reaction mixture was allowed to come to room temperature and then heated at about 140 0C for about 24 hours. The reaction mixture was cooled, poured into chilled water and extracted with ethyl acetate to form a product in 38 % yield. The above product was dissolved in methanol, 10 % dry palladium-carbon was added and hydrogenated in Parr apparatus under about 50-60 psi pressure. The reaction mixture was filtered through a bed of celite, the bed washed well with methanol, filtrate concentrated to afford the product in 94 % yield. Equimolar quantities of the above compound and Bis-(2- chloroethyl)amine hydrochloride were heated in a mixture of 1,2-dichlorobenzene and n- hexanol (10: 1) for about 42 hours at about 1600C. The reaction mixture was cooled to about 400C poured into hexane, filtered to form l-[5-fluoro-2-(2,2,2- trifluoroethoxy)pheny I] D iperazines. Yield: 85 %
References:
WO2006/117760,2006,A1 Location in patent:Page/Page column 49-50

334929-98-5
7 suppliers
inquiry

7440-02-0
427 suppliers
$14.39/1slug

334929-99-6
18 suppliers
$110.00/50mg