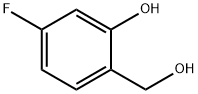
5-fluoro-2-(hydroxymethyl)phenol synthesis
- Product Name:5-fluoro-2-(hydroxymethyl)phenol
- CAS Number:773873-09-9
- Molecular formula:C7H7FO2
- Molecular Weight:142.13
345-29-9
223 suppliers
$10.00/1g

773873-09-9
14 suppliers
inquiry
Yield:773873-09-9 96%
Reaction Conditions:
Stage #1: 4-fluorosalicylic acidwith lithium aluminium tetrahydride in tetrahydrofuran at 0 - 20; for 60 h;
Stage #2: with hydrogenchloride in tetrahydrofuran;water at 0 - 20;
Steps:
1; 53
To an ice bath-cooled solution of lithium aluminiumhydride (1 M in THF, 25 ml, 25 mmol) a solution of 4-fluoro-2-hydroxybenzoic acid (1.56 g, 10.0 mmol) in THF (10 ml) was added drop-wise. The mixture was allowed to warm up to room temperature and after stirring over night cooled with an ice bath and quenched carefully with aqueous 2M HCl (approx. 30 ml). The mixture was extracted twice with ethyl acetate, the combined organic phases were washed with aqueous 1 M sodium bicarbonate (50 ml) and dried over sodium sulfate. Evaporation of the solvent afforded 1.37 g (96 %) of Id as a brown solid. 1H NMR (400 MHz, CDCl3) δ 7.58 (br.s, IH), 6.98 (dd, J= 8.2, 6.5 Hz, IH), 6.63 (dd, J= 10.2, 2.5 Hz, IH), 6.56 (td, J= 8.3, 2.5 Hz, IH), 4.85 (s, 2H), 2.05 (s, IH). Example 53. To a cold (ice- water bath) solution of LiAlH4 in THF (10 ml, 10 mmol) was added a solution of 4-fiuoro-2-hydroxybenzoic acid (624 mg, 4 mmol) in THF (5 ml) slowly. After addition was completed ice bath was removed and the reaction mixture was stirred at room temperature for about 21A day, cooled to 0 0C5 dil. aqueous HCl (20 ml) was added carefully. The ice bath was removed and after 40 min at room temperature the reaction mixture was extracted with ethyl acetate. The organic layer was washed with water and aqueous NaHCO3 respectively, dried over Na2SO4, filtered and the filtrate was concentrated in vacuo. The residue was dissolved in DMF (3 ml), NaCN (230 mg, 4.69 mmol) was added and the reaction mixture was stirred at 120 °C for 4.5 h, cooled to room temperature and extracted with ethyl acetate. The organic layer was washed with aqueous NH4Cl, dried over Na2SO4, filtered and the filtrate was concentrated in vacuo. The residue was purified by silica gel flash chromatography (0-20% ethyl acetate in petroleum spirit 40-60) to give subtitle compound (180 mg). 1H-NMR (DMSO-d6, 400 MHz): δ 10.60 (br.s, IH); 7.26 (t, J= 8.0 Hz, IH); 6.64 (m, 2H); 3.68 (s, 2H).
References:
WO2007/53082,2007,A1 Location in patent:Page/Page column 33