
5-Fluoro-2-iodo-4-methyl-phenylamine synthesis
- Product Name:5-Fluoro-2-iodo-4-methyl-phenylamine
- CAS Number:1126423-32-2
- Molecular formula:C7H7FIN
- Molecular Weight:251.04
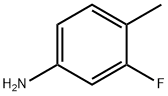
452-77-7
265 suppliers
$8.00/5g

1126423-32-2
14 suppliers
inquiry
Yield:1126423-32-2 95%
Reaction Conditions:
with N,N,N-trimethylbenzenemethanaminium dichloroiodate;calcium carbonate in methanol;dichloromethane at 0 - 20; for 17 h;
Steps:
12.1
Example 12; Preparation of Compound 160, 161 and 162; Step 1 - Synthesis of Compound 12B
References:
WO2009/32124,2009,A1 Location in patent:Page/Page column 168-169