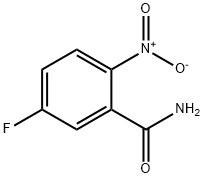
5-FLUORO-2-NITROBENZAMIDE synthesis
- Product Name:5-FLUORO-2-NITROBENZAMIDE
- CAS Number:77206-97-4
- Molecular formula:C7H5FN2O3
- Molecular Weight:184.12
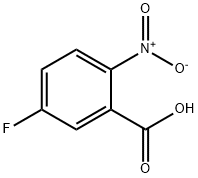
320-98-9
284 suppliers
$8.00/5g

77206-97-4
27 suppliers
inquiry
Yield:77206-97-4 96%
Reaction Conditions:
with ammonia;(benzotriazo-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate;N-ethyl-N,N-diisopropylamine in 1,4-dioxane;dichloromethane at 20; for 16 h;Inert atmosphere;
Steps:
1.1 Step 1 :
Under inert atmosphere, a solution of 5-fluoro-2-nitrobenzoic acid (2.50 g, 13.5 mmol), ammonia (0.5M in dioxane, 54.0 ml_, 27.0 mmol), benzotriazol-1-yloxy- tris(dimethylamino)-phosphonium hexafluorophosphate (8.90 g, 20.3 mmol) and diisopropylethylamine (6.10 ml_, 35.1 mmol) in anhydrous dichloromethane (68.0 ml.) was stirred for 16 h at room temperature. Then, the mixture was poured into an aqueous saturated solution of ammonium chloride (250 ml.) and extracted with dichloromethane (2 x 200 ml_). The combined organic extracts were washed with brine (100 ml_), dried over MgS04 and concentrated under vacuum. The crude dark solid was purified by flash column chromatography on silica gel using cyclohexane / ethyl acetate as eluent to afford 5-fluoro-2-nitrobenzamide (2.40 g, 13.0 mmol, 96%) as a brown solid. (0777) M/Z (M+H)+ = 185.2.
References:
WO2020/21064,2020,A1 Location in patent:Page/Page column 103
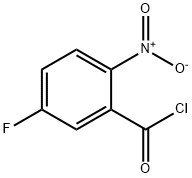
394-02-5
28 suppliers
$13.00/250mg

77206-97-4
27 suppliers
inquiry
455-38-9
384 suppliers
$11.92/25g

77206-97-4
27 suppliers
inquiry