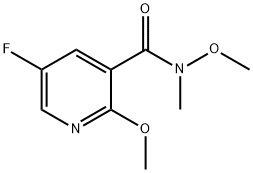
5-FLUORO-N,2-DIMETHOXY-N-METHYLNICOTINAMIDE synthesis
- Product Name:5-FLUORO-N,2-DIMETHOXY-N-METHYLNICOTINAMIDE
- CAS Number:1346817-38-6
- Molecular formula:C9H11FN2O3
- Molecular Weight:214.19
884494-82-0
113 suppliers
$30.00/100mg

6638-79-5
553 suppliers
$6.00/25g

1346817-38-6
5 suppliers
inquiry
Yield:1346817-38-6 43%
Reaction Conditions:
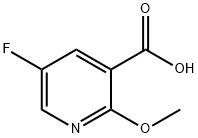
Stage #1: 5-fluoro-2-methoxy-pyridine-3-carboxylic acidwith 1,1'-carbonyldiimidazole in tetrahydrofuran at 20; for 0.416667 h;
Stage #2: N,O-dimethylhydroxylamine*hydrochloride in tetrahydrofuran at 20; for 72 h;
Steps:
4
PREPARATION 45-Fluoro-2,N-dimethoxy-N-methyl-nicotinamideTo a solution of 5-fluoro-2-methoxynicotinic acid (1 g, 5.8 mmol) in tetrahydrofuran (10 mL) was added 1 , 1 '-carbonyldiimidazole (1.4 g, 8.77 mmol, 1.5 eq.) portionwise over a 5 min period. The resulting solution was stirred at room temperature for 20 min before adding O, N-Dimethyl-hydroxylamine hydrochloride (0.63 g, 6.43 mmol, 1.1 eq.) and then stirred for a further 72 hr. The resulting mixture was then partitioned between ethyl acetate (30 mL) and 2M aqueous hydrochloric acid (30 mL). The organic phase was washed with 1 M aqueous sodium hydrogen carbonate (30 ml_), followed by brine (30 ml_) and then dried over magnesium sulphate. The resulting mixture was filtered and concentrated under reduced pressure to produce the title compound as a colourless oil (0.53 g, 43%).1H NMR (400 MHz, CDCI3): δ ppm 3.36 (br. s., 3 H) 3.55 (br. s., 3 H) 3.97 (s, 3 H) 7.33 - 7.44 (m, 1 H) 8.06 (d, J=2.73 Hz, 1 H).LRMS: APCI, m/z = 215 [M+H]+
References:
WO2011/138751,2011,A2 Location in patent:Page/Page column 68-69