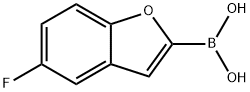
(5-Fluorobenzofuran-2-yl)boronic acid synthesis
- Product Name:(5-Fluorobenzofuran-2-yl)boronic acid
- CAS Number:473416-33-0
- Molecular formula:C8H6BFO3
- Molecular Weight:179.94
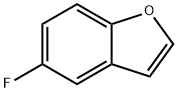
24410-59-1
82 suppliers
$60.00/100mg

5419-55-6
373 suppliers
$12.00/5g

473416-33-0
46 suppliers
inquiry
Yield:473416-33-0 26%
Reaction Conditions:
Stage #1: 5-Fluoro[b]benzofuranwith n-butyllithium;N,N,N,N,-tetramethylethylenediamine in tetrahydrofuran;hexane at -60 - -10; for 75 h;Inert atmosphere;
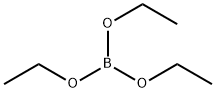
Stage #2: Triisopropyl borate in tetrahydrofuran;hexane at -60 - 20;Inert atmosphere;
Stage #3: with hydrogenchloride;water in tetrahydrofuran;hexane;
Steps:
4.3
Step 3. 5-Fluorobenzofuran-2-ylboronic acid To a solution of 5-fluorobenzofuran (10 g, 73.53 mmol) in dry tetrahydrofuran (250 mL) was added tetramethylethylenediamine (10.2 g, 87.93 mmol). The solution was kept below -60° C. under nitrogen, while BuLi (93.75 mmol, 2.5M solution in hexane) was added dropwise. It was warmed to -10° C. during 45 min and stirred at this temperature for another 30 min. The mixture was cooled again below -60° C. followed by dropwise addition of triisopropyl borate (41.4 g, 220.21 mmol). After warming to room temperature the mixture was quenched with hydrochloric acid (70 mL, 2N) and stirred for 1 h. The alkaline aqueous layer was brought to pH 5 and extracted with ethyl acetate (3×80 mL). All organic layers were combined, dried over sodium sulfate, and concentrated in vacuo to give 5-fluorobenzofuran-2-ylboronic acid (3.5 g, 26%) which was used for the next step without further purification.1H-NMR (300 MHz, CDCl3): δ 8.63 (s, 2H), 7.58-7.62 (m, 1H), 7.44-7.49 (m, 2H), 7.15-7.22 (m, 1H)
References:
US2012/225863,2012,A1 Location in patent:Page/Page column 17-18

24410-59-1
82 suppliers
$60.00/100mg
150-46-9
189 suppliers
$14.00/25mL

473416-33-0
46 suppliers
inquiry

24410-59-1
82 suppliers
$60.00/100mg

5419-55-6
373 suppliers
$12.00/5g

7732-18-5
464 suppliers
$13.50/100ML

473416-33-0
46 suppliers
inquiry

59769-37-8
12 suppliers
$195.00/1g

473416-33-0
46 suppliers
inquiry

371-41-5
512 suppliers
$8.00/25g

473416-33-0
46 suppliers
inquiry