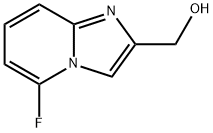
(5-FLUOROIMIDAZO[1,2-A]PYRIDIN-2-YL)METHANOL synthesis
- Product Name:(5-FLUOROIMIDAZO[1,2-A]PYRIDIN-2-YL)METHANOL
- CAS Number:878197-92-3
- Molecular formula:C8H7FN2O
- Molecular Weight:166.15
![5-FLUOROIMIDAZO[1,2-A]PYRIDINE-2-CARBOXALDEHYDE](/CAS/GIF/878197-67-2.gif)
878197-67-2
36 suppliers
$45.00/10mg
![(5-FLUOROIMIDAZO[1,2-A]PYRIDIN-2-YL)METHANOL](/CAS/GIF/878197-92-3.gif)
878197-92-3
14 suppliers
inquiry
Yield:878197-92-3 93%
Reaction Conditions:
Stage #1: 5-fluoroimidazo[1,2-a]pyridine-2-carbaldehydewith sodium tetrahydroborate in methanol at 0 - 20; for 2 h;
Stage #2: with water in methanol;
Steps:
13.D
D) (5-FluoroimidazoM .2-alPyridin-2-yl)methanol: A solution of 5-fluoroimidazo[1 ,2-a]pyridine-2-carbaldehyde (80 g, 490 mmol) in methanol (1 L) at O0C was treated with sodium borohydride (24 g, 640 mmol) in portions. The reaction was slowly brought to room temperature, stirred for 2 hours, quenched with water, concentrated, dissolved in 3:1 dichloromethane to isopropyl alcohol, and washed with saturated aqueous sodium bicarbonate. The organic layer was separated and the aqueous extracted four times with 3:1 dichloromethane to isopropyl alcohol. The organic layers were combined, dried over sodium sulfate, concentrated, triturated with hexanes, and filtered to yield (5-fluoroimidazo[1,2-a]pyridin-2-yl)methanol (76 g, 93% yield) as a brown solid. 1H-NMR (CDCI3): δ 7.59 (s, 1 H), 7.38 (d, 1H), 7.21-7.15 (m, 1 H), 6.43 (m, 1H), 4.85 (S, 2H), 4.45 (s, 1H).
References:
WO2006/76131,2006,A2 Location in patent:Page/Page column 41