
5-HYDROXY-2-(HYDROXYMETHYL)-4-PYRIDONE synthesis
- Product Name:5-HYDROXY-2-(HYDROXYMETHYL)-4-PYRIDONE
- CAS Number:31883-16-6
- Molecular formula:C6H7NO3
- Molecular Weight:141.12
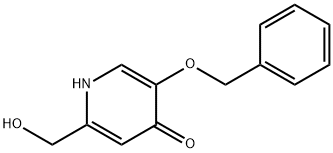
59281-14-0
29 suppliers
$49.00/1g

31883-16-6
26 suppliers
inquiry
Yield: 82.1%
Reaction Conditions:
with hydrogenchloride;5%-palladium/activated carbon;hydrogen in methanol at 20; under 1551.49 Torr; for 8 h;
Steps:
Synthesis of5-hydroxy-2-(hydroxymethyl)pyridin-4(1H)-one (4)
To a suspension of compound3 (10 g, 43.24 mol) in MeOH (50 mL)was added concentrated hydrochloric acid (4 mL) and 5% Pd/C (0.5 g).Hydrogenation was carried out under 30 psi H2 for 8 h at roomtemperature. After filtration to remove the catalyst, the filtrate wasconcentrated to dryness. The residue was redissolved in methanol (15mL), NaHCO3solution (10%) was added until no bubbles appeared. Compound 4 was obtained as a white powder byfiltration (5.0 g, 82.1% yield). 1H NMR (500MHz, DMSO-d6)δ: 4.34 (s, 2H, CH2), 6.15 (s, 1H, C3-H in pyridinone), 7.27 (s, 1H,C6-H in pyridinone). 13CNMR (125MHz, DMSO-d6) δ: 59.7, 108.6, 117.4, 146.3, 147.5, 170.7.ESI-HRMS: m/z, calcd for C6H7NO3 ([M+H]+) 141.0426,found 141.0431.
References:
Zhao, De-Yin;Zhang, Ming-Xia;Dong, Xiao-Wu;Hu, Yong-Zhou;Dai, Xiao-Yan;Wei, Xiaoyi;Hider, Robert C.;Zhang, Jin-Chao;Zhou, Tao [Bioorganic and Medicinal Chemistry Letters,2016,vol. 26,# 13,p. 3103 - 3108] Location in patent:supporting information

501-30-4
612 suppliers
$5.00/25mg

31883-16-6
26 suppliers
inquiry
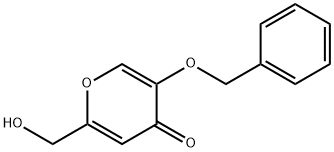
15771-06-9
55 suppliers
$25.00/250mg

31883-16-6
26 suppliers
inquiry