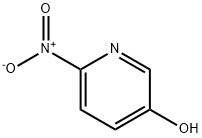
5-HYDROXY-2-NITROPYRIDINE synthesis
- Product Name:5-HYDROXY-2-NITROPYRIDINE
- CAS Number:15206-26-5
- Molecular formula:C5H4N2O3
- Molecular Weight:140.1
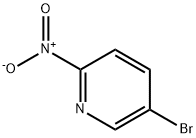
39856-50-3
520 suppliers
$6.00/5g

15206-26-5
119 suppliers
inquiry
Yield: 21.8%
Reaction Conditions:
Stage #1:5-bromo2-nitropyridine with potassium acetate;palladium diacetate;bis(pinacol)diborane in N,N-dimethyl-formamide at 100; for 16 h;
Stage #2: with sodium perborate tetrahydrate in tetrahydrofuran;water at 20; for 16 h;
Steps:
2
A suspension of 5-bromo-2-nitro-pyridine (10.0 g, 49 mmol), Boric acid ester (13.7 g, 54 mmol), Pd (OAc)2 (121 mg, 0.54 mmol) and KOAc (14.4 g, 147 mmol) in DMF (180 mL) was heated to 100 °C for 16 h. After the solvent was removed under vacuum, the remaining residue was mixed with EtOAc (600 mL). The EtOAc solution was washed with water (100 mL), brine (100 mL), and dried over Na2S04. It was concentrated to a residue that was mixed with NaBOs 4H20 (19.0g, 125 mmol), THF (180 mL) and H20 (180 mL). The resulting mixture was stirred at room temperature for 16 h. The aqueous phase was separated with the organic phase, and washed with EtOAc (100 mL x 2). The combined organic solution was then concentrated to give 6-nitro-pyridin-3-ol (1.5 g, 21.8 %) as a yellow solid.
References:
MANNKIND CORPORATION;TOLERO PHARMACEUTICALS, INC.;ZENG, Qingping;FARIS, Mary;MOLLARD, Alexis;WARNER, Steven L.;FLYNN, Gary A. WO2014/52365, 2014, A1 Location in patent:Page/Page column 168
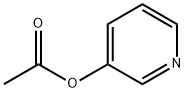
17747-43-2
69 suppliers
$41.00/5g

15206-26-5
119 suppliers
inquiry