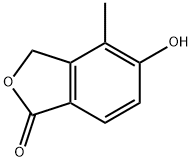
5-hydroxy-4-methyl-2-benzofuran-1(3H)-one synthesis
- Product Name:5-hydroxy-4-methyl-2-benzofuran-1(3H)-one
- CAS Number:1194700-73-6
- Molecular formula:C9H8O3
- Molecular Weight:164.16
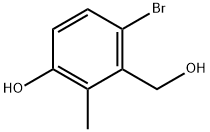
1255206-72-4
11 suppliers
inquiry

1194700-73-6
48 suppliers
$62.00/250mg
Yield:-
Reaction Conditions:
with copper(I) cyanide in N,N-dimethyl-formamide at 145;Inert atmosphere;
Steps:
3B.C Step C: 5-Hydroxy-4-methyl-3H-isobenzofuran-1-one
Step C: 5-Hydroxy-4-methyl-3H-isobenzofuran-1-one; To a 2 L 3 neck flask equipped withoverhead stirrer, N2 inlet, and condenser were charged 4-bromo-3-hydroxymethyl-2-methyl phenol (100 g, 461 mmol), CuCN (83.0 g, 921 mmol), and DMF (500 mL). The solution was phenol (100 g, 461 mmol), CuCN (83.0 g, 921 mmol), and DMF (500 mL). The solution wassparged with N2 for 15 min then heated to 145 °C to obtain a homogeneous solution. Thesolution was aged at 145 °C for 2h, then the reaction mixture was cooled to 95 °C. 41.5 mLwater was added (sparged with N2), and the reaction aged for 20 h. The reaction was cooled toRT then the solids filtered through solka flok and the cake washed with 50 mL DMF. To a 3 L flask containing 1 L EtOAc was added the DMF filtrate. A precipitate coating formed in bottomof flask. The DMF/EtOAc suspension was filtered through solka flok and the cake was washedwith 250 mL EtOAc. The resulting filtrate was washed with 5% brine solution (3 x 500 mL).The aqueous layers were extracted with 500 mL EtOAc and the combined organics were driedover MgS04, fitered and evaporated. The solids were slurried in 250 mL MTBE at RT then filtered and washed with 100 mL MTBE. The solids were dried under vaccum at RT, providing5-hydroxy-4-methyl-3H-isobenzofuran-1-one.
References:
WO2013/28474,2013,A1 Location in patent:Page/Page column 30
201230-82-2
1 suppliers
inquiry

1255206-72-4
11 suppliers
inquiry

1194700-73-6
48 suppliers
$62.00/250mg

1255206-72-4
11 suppliers
inquiry
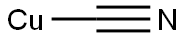
544-92-3
251 suppliers
$9.00/1g

1194700-73-6
48 suppliers
$62.00/250mg
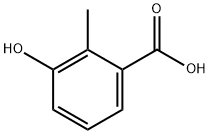
603-80-5
267 suppliers
$14.00/1g

1194700-73-6
48 suppliers
$62.00/250mg

54874-26-9
34 suppliers
inquiry

1194700-73-6
48 suppliers
$62.00/250mg