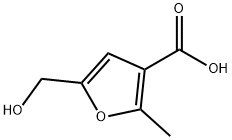
5-(HYDROXYMETHYL)-2-METHYL-3-FUROIC ACID synthesis
- Product Name:5-(HYDROXYMETHYL)-2-METHYL-3-FUROIC ACID
- CAS Number:15341-68-1
- Molecular formula:C7H8O4
- Molecular Weight:156.14
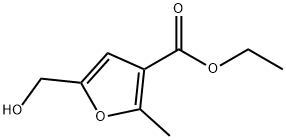
15341-70-5
0 suppliers
inquiry

15341-68-1
11 suppliers
inquiry
Yield:15341-68-1 95%
Reaction Conditions:
with ethanol;sodium hydroxide at 60; for 5.5 h;
Steps:
5-Hydroxymethyl-2-methylfuran-3-carboxylic acid (39)
To a solution of 38 (1 .64 g, 9.01 mmol) in MeOH, NaBH4 (682 mg, 18.02 mmol) was added. The mixture was stirred at room temperature for 30 min. The mixture was neutralized with citric acid. After evaporation of solvent, the residue was diluted with CH2CI2 and washed with water. The organic phases were dried, filtered and concentrated to give ethyl 5-hydroxymethyl-2-methylfuran-3-carboxylic ester (1 .39 g, 7.54 mmol, 84 %, MW: 184.191 ) as a white solid. IR (v cm 1) 3423 (OH), 1710 (C=0). 1H NMR (300 MHz, CDCI3, δ ppm, J Hz) δ 6.52 (s, 1 H, H-3), 4.53 (s, 1 H, H-f), 4.26 (q, 2H, 2JH,H = 6.9, - CH2CH3), 2.55 (s, 3H, Me), 1 .33 (t, 3H, -CH2CH3). 13C NMR (75.4 MHz, CDCI3, δ ppm) δ 177.2 (CHO), 164.8 (COOEt), 162.7 (C-5), 150.4 (C-2), 122.6 (C-3), 1 16.6 (C-4), 61 .0 (- CH2CH3), 14.4 (Me), 14.4 (-CH2CH3). CIMS m/z 185 [46%, (M+H)+], 184 [61 %, (M)+], 167 [100%, (M-OH+H)+]. HRCIMS m/z found 184.0732, calc. for C9H1204: 184.0736. This ester (1 .36 mg, 7.41 mmol) was dissolved in 1 M NaOH/EtOH (30 mL) and the mixture was stirred at 60QC for 5.5 h. The mixture was cooled to 0 QC, neutralized with acid resin IR 120 (H+), filtered and concentrated to give 39 (1 .1 Og, 7.04 mmol, 95 %, MW: 156.137) as a white solid. IR (v cm 1) 3330 (OH), 1665 (C=0). 1H NMR (300 MHz, MeOD, δ ppm, J Hz) δ 6.50 (s, 1 H, H-3), 4.45 (s, 1 H, H-1 '), 2.54 (s, 3H, Me). 13C NMR (75.4 MHz, MeOD, δ ppm) δ 167.3 (-COOH), 160.4 (C-5), 154.1 (C-2), 1 15.4 (C-4), 109.4 (C-3), 57.1 (C-f), 13.7 (Me). CIMS m/z 158 [63%, (M+2H)+], 157 [65%, (M+H)+], 139 [100%, (M-OH+H)+]. HRCIMS m/z found 157.0501 , calc. for C7H904: 157.0501 .
References:
WO2018/24907,2018,A1 Location in patent:Page/Page column 64; 65
![3-Furancarboxylic acid, 2-methyl-5-[(1S,2R,3R)-1,2,3,4-tetrahydroxybutyl]-, ethyl ester](/CAS/20210305/GIF/3351-43-7.gif)
3351-43-7
0 suppliers
inquiry

15341-68-1
11 suppliers
inquiry
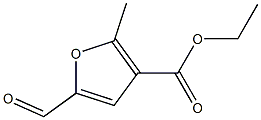
19615-48-6
1 suppliers
inquiry

15341-68-1
11 suppliers
inquiry
![Ethanone, 1-[5-(hydroxymethyl)-2-methyl-3-furanyl]-](/CAS/20180601/GIF/70107-20-9.gif)
70107-20-9
0 suppliers
inquiry

15341-68-1
11 suppliers
inquiry
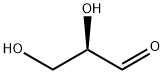
453-17-8
109 suppliers
$32.00/100mg
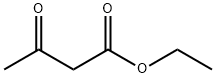
141-97-9
784 suppliers
$5.00/25g

15341-68-1
11 suppliers
inquiry