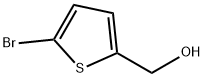
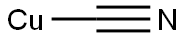5-(HydroxyMethyl)thiophene-2-carbonitrile synthesis
- Product Name:5-(HydroxyMethyl)thiophene-2-carbonitrile
- CAS Number:172349-09-6
- Molecular formula:C6H5NOS
- Molecular Weight:139.18
21512-16-3
91 suppliers
$8.00/100mg

172349-09-6
31 suppliers
inquiry
Yield: 6.1 g (88%)
Reaction Conditions:
with sodium borohydrid in ethanol
Steps:
61.B B)
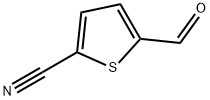
B) Preparation of 2-cyano-5-(hydroxymethyl)thiophene To a solution of 2-cyano-5-formyl-thiophene (6.9 g, 50 mmol) in EtOH (100 mL) was added sodium borohydride (1.9 g, 50 mmol) in portions. After 5 min of stirring, the solvent was removed in vacuo and the residue was partitioned between ethyl acetate and brine. The layers were separated and the organic phase was washed once with 1M citric acid and once with brine, then dried (MgSO4), filtered and concentrated in vacuo to give 6.1 g (88%) of 2-cyano-5-(hydroxymethyl)thiophene. 1 H NMR FD-MS, m/e 140 (M+) Analysis for C6 H5 NOS: Calc: C, 51.78; H, 3.62; N, 10.06; Found: C, 51.54; H, 3.62; N, 9.86.
References:
Eli Lilly and Company US5914319, 1999, A

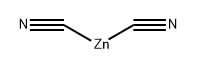
4701-17-1
305 suppliers
$6.00/1g

172349-09-6
31 suppliers
inquiry