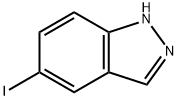
5-Iodo-1H-indazole synthesis
- Product Name:5-Iodo-1H-indazole
- CAS Number:55919-82-9
- Molecular formula:C7H5IN2
- Molecular Weight:244.03

19335-11-6
306 suppliers
$10.00/1 g

55919-82-9
147 suppliers
$6.00/1g
Yield:55919-82-9 100%
Reaction Conditions:
Stage #1:1H-indazol-5-ylamine with hydrogenchloride;sodium nitrite in water at -5 - 2; for 1.25 h;
Stage #2: with potassium iodide in water at 90; for 1.75 h;
Steps:
1
SYNTHETIC PREPARATION 15-Aminoindazole (64.73 g, 486.13 mmol) was suspended in 600 mL water and ca. 600 mL ice, and cone. HCI ( 200 mL, 5759 mmol) was added. The mixture was cooled in an ice-salt bath to ca. -5 0C. To this mixture was added, dropwise, a solution of sodium nitrite (37.34 g, 541.2 mmol) in 200 mL water (took about 1 hr). The internal temperature was kept below ca. +2 0C. The resulting brown solution was stirred for a further 15 min at -5 0C, then a solution of potassium iodide (97 g, 584.34 mmol) in 250 mL water was slowly added dropwise (took about 30 min). After complete addition, the reaction was heated to 90 0C for 1.5 hr. After allowing to cool, the solution was filtered to give a fine black solid and the filtrate was allowed to sit overnight in the refrigerator. The next day the filtrate was filtered again and the two solids were combined and dried to give 5-iodoindazole (126.63 g, 106%).
References:
BAYER SCHERING PHARMA AKTIENGESELLSCHAFT WO2008/71451, 2008, A1 Location in patent:Page/Page column 47
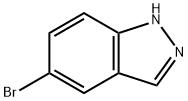
53857-57-1
359 suppliers
$6.00/1g

55919-82-9
147 suppliers
$6.00/1g
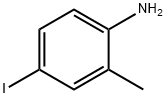
13194-68-8
205 suppliers
$6.00/1g

55919-82-9
147 suppliers
$6.00/1g

850363-46-1
2 suppliers
inquiry

55919-82-9
147 suppliers
$6.00/1g
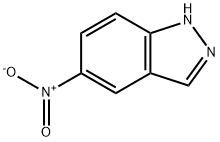
5401-94-5
369 suppliers
$14.00/5g

55919-82-9
147 suppliers
$6.00/1g