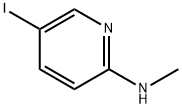
(5-Iodo-pyridin-2-yl)-methyl-amine synthesis
- Product Name:(5-Iodo-pyridin-2-yl)-methyl-amine
- CAS Number:280116-78-1
- Molecular formula:C6H7IN2
- Molecular Weight:234.04
4597-87-9
168 suppliers
$17.00/1g

280116-78-1
9 suppliers
inquiry
Yield:280116-78-1 62%
Reaction Conditions:
with Iodine monochloride in dichloromethane at 0; for 0.5 h;
Steps:
15
2-(N-Monomethylamino)pyridine (98, 1.00 g, 5.55 mmol) was dissolved in dichloromethane (20 mL) and a 1 M iodine monochloride (ICl, 6.10 mL, 6.10 mmol) dichloromethane solution was slowly added at 0° C., and stirred for 30 minutes. To the reaction mixture was added a 10% aqueous solution of sodium sulfite (Na2S2O3) and organic compounds were extracted with dichloromethane and evaporated after a treatment with sodium sulfate. Purification was performed by column chromatograph to give the target compound 2-(N-monomethylamino)-5-iodopyridine (99, 800 mg, 62%) as a yellow solid.1H NMR (400 MHz, CDCl3) δ 2.88 (s, 3H), 4.57 (s, 1H), 6.24 (d, J=8.4 Hz, 1H), 7.62 (dd, J=8.8, 2.4 Hz, 1H), 8.23 (d, J=2.4 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 28.9, 76.2, 108.5, 144.8, 153.8, 158.3
References:
US2010/261727,2010,A1 Location in patent:Page/Page column 23-24
![Pyridinium, 1-[(5-iodo-2-pyridinyl)amino]-, inner salt](/CAS/20211123/GIF/157020-27-4.gif)
157020-27-4
0 suppliers
inquiry

280116-78-1
9 suppliers
inquiry