
5-isopropoxy-2-methyl-1H-indole synthesis
- Product Name:5-isopropoxy-2-methyl-1H-indole
- CAS Number:1134334-84-1
- Molecular formula:C12H15NO
- Molecular Weight:189.25
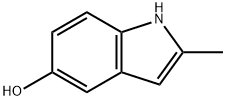
13314-85-7
142 suppliers
$22.00/1g
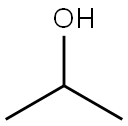
67-63-0
1554 suppliers
$17.00/25ML

1134334-84-1
6 suppliers
$189.00/500mg
Yield:1134334-84-1 69%
Reaction Conditions:
with triphenylphosphine;diethylazodicarboxylate in dichloromethane at 20;
Steps:
4.1.28. 5-Isopropoxy-2-methyl-1H-indole (44)
At RT, diethyl azodicarboxylate (DEAD, 0.672 mL, 4.27 mmol) was slowly added to a solution of 2-methyl-1H-indol-5-ol (43, 0.42 g, 2.85 mmol), Ph3P (1.49 g, 5.7 mmol) and propan-2-ol (0.214 g, 3.56 mmol), in 40 mL of CH2Cl2. The mixture was stirred at RT until the reaction was complete (TLC analysis) and then quenched with methanol (3 mL).Water was added and the mixture was extracted with CH2Cl2. The organic extracts were washed with water and brine, dried over MgSO4, and concentrated to give the crude product. Purification was performed using flash chromatography on silica gel (isooctane/ethyl acetate, gradient) to give the title compound in 69% yield (0.31 g, 1.63 mmol). MS m/z (relative intensity, 70 eV) 189 (M+, 40), 147 (bp), 146 (72), 118 (13), 117 (11).ESIMS: m/z 190.0 (M + H)+. 1H NMR (CD3OD, 300 MHz) δ: 1.26 (d, J = 6 Hz, 6H), 2.36 (s, 3H), 4.42 (pentamer, J = 6 Hz, 1H), 6.01 (s, 1H), 6.64 (dd, J = 8.7, 2.4 Hz, 1H), 6.94 (d, J = 2.4 Hz, 1H), 7.11 (d, J = 8.4 Hz, 1H). 13C NMR (CD3OD, 75 MHz) δ: 13.52, 22.59, 72.95, 100.18, 107.30, 111.66, 113.36, 131.01, 133.63, 137.42, 152.46.
References:
Mattsson, Cecilia;Svensson, Peder;Boettcher, Henning;Sonesson, Clas [European Journal of Medicinal Chemistry,2013,vol. 63,p. 578 - 588]
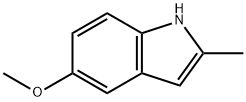
1076-74-0
192 suppliers
$15.00/1g

1134334-84-1
6 suppliers
$189.00/500mg