
5-METHOXY-1,3-BENZOTHIAZOL-2-AMINE synthesis
- Product Name:5-METHOXY-1,3-BENZOTHIAZOL-2-AMINE
- CAS Number:54346-87-1
- Molecular formula:C8H8N2OS
- Molecular Weight:180.23
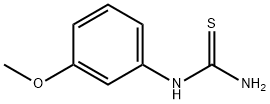
37014-08-7
70 suppliers
$24.00/1g

54346-87-1
116 suppliers
$17.00/250mg
Yield: 95%
Reaction Conditions:
with bromine in dichloromethane;chloroform at 20; for 4 - 6 h;Heating / reflux;
Steps:
3.I
I. N-(5-tert-butyl-isoxazol-3-yl)-N'-{4-[6-(2-morpholin-4-yl-ethoxy)imidazo[2,1-b][1,3]benzothiazol-2-yl]phenyl}urea hydrochloride [Compound B12] was also prepared by first preparing the benzothiazole starting material, 5 methoxy-benzothiazol-2-yl-amine: To prepare the 5-methoxy-benzothiazol-2-ylamine starting material: To a suspension of (3-methoxy-phenyl)-thiourea (1.822 g, 10 mmol) in CH2Cl2 (20 mL) at 0 C. was added dropwise a solution of bromine (1.76 g, 11 mmol) in 10 ml of trichloromethane over a period of thirty minutes. The reaction was stirred for 3 hours at room temperature then heated to 3 hours to reflux for one hour. The precipitate was filtered and washed with dichloromethane. The solid was suspended in saturated NaHCO3 and extracted with CH2Cl2. The extract was dried over MgSO4 and concentrated to give a white solid (1.716 g, 95%). To prepare the 2-amino-benzothiazol-5-ol: To a suspension of 5-methoxy-benzothiazol-2-ylamine in 16 mL of 48% HBr/H2O was heated to 105 C. in an oil bath for 10 hours. After the reaction was cooled to room temperature, the precipitate was collected by filtration and washed with acetone. The filtrate was suspended in saturated NaHCO3 and extracted with CH2Cl2. The extract was dried over MgSO4 and concentrated to give a white solid (0.986 g, 63%). N-(5-tert-butyl-isoxazol-3-yl)-N'-{4-[6-(2-morpholin-4-yl-ethoxy)imidazo[2,1-b][1,3]benzothiazol-2-yl]phenyl}urea hydrochloride 2-amino-benzothiazol-5-ol from the previous step and following the method described in 1H NMR (DMSO-d6) ? 11.1 (br, 1H), 9.69 (br, 1H), 9.28 (br, 1H), 8.71 (s, 1H), 7.97 (d, 1H), 7.79 (d and s, 3H), 7.56 (d, 2H), 7.13 (dd, 1H), 6.53 (s, 1H), 4.56 (t, 2H), 3.98 (m, 2H), 3.82 (t, 2H), 3.65 (m, 2H), 3.55 (m, 2H), 3.25 (m, 2H), 1.31 (s, 9H); LC-MS: ESI 561 (M+H)+. [Compound B12]
References:
Bhagwat, Shripad;Chao, Qi;Grotzfeld, Robert M.;Patel, Hitesh K.;Sprankle, Kelly G. US2007/232604, 2007, A1 Location in patent:Page/Page column 40
27191-09-9
50 suppliers
$10.00/1g

54346-87-1
116 suppliers
$17.00/250mg

536-90-3
303 suppliers
$10.00/5g

54346-87-1
116 suppliers
$17.00/250mg
333-20-0
444 suppliers
$13.50/100G

536-90-3
303 suppliers
$10.00/5g

54346-87-1
116 suppliers
$17.00/250mg