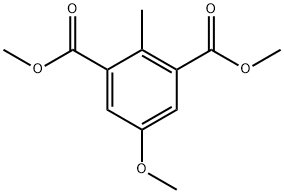
5-Methoxy-2-methyl-isophthalic acid synthesis
- Product Name:5-Methoxy-2-methyl-isophthalic acid
- CAS Number:13979-74-3
- Molecular formula:C12H14O5
- Molecular Weight:238.24

67-56-1
738 suppliers
$9.00/25ml
201230-82-2
1 suppliers
inquiry
14542-71-3
86 suppliers
$25.00/250mg

13979-74-3
3 suppliers
inquiry
Yield:13979-74-3 93%
Reaction Conditions:
with (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride;triethylamine at 100; under 6000.6 Torr; for 40 h;Sealed tube;
Steps:
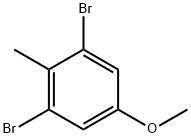
2 Step 2: dimethyl 5-methoxy-2-methylbenzene-1,3-dicarboxylate
A mixture of 1,3-dibromo-5-methoxy-2-methylbenzene (7a) (12.3 g, 43.9 mmol), PdCI2(dppf) (2.01 g, 2.75 mmol) and TEA (13.3 g, 132 mmol) in MeOH (350 ml_, c = 0.13 M) was sealed under an atmosphere of CO (0.8 MPa) and stirred at 100 °C for 40 h. TLC analysis (petroleum ether) indicated consumption of the starting material. The reaction mixture was combined with a parallel reaction conducted with 900 mg 1,3-dibromo-5-methoxy-2-methylbenzene. The mixture was filtered. The filter cake was washed with MeOH (3x10 mL). The filtrate was concentrated to dryness to provide a red solid. The residue was purified by flash chromatography (Isco, 120 g S1O2, petroleum ether/EtOAc = 6:1) to provide dimethyl 5-methoxy- 2-methylbenzene-1,3-dicarboxylate (10.5 g, 93% yield) as a colorless oil. 1H NMR (400 MHz, CDC) d 7.41 (s, 2H), 3.94 - 3.88 (m, 6H), 3.83 (s, 3H), 2.59 (s, 3H).
References:
WO2021/224818,2021,A1 Location in patent:Page/Page column 46-47