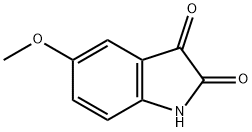
5-Methoxyisatin synthesis
- Product Name:5-Methoxyisatin
- CAS Number:39755-95-8
- Molecular formula:C9H7NO3
- Molecular Weight:177.16
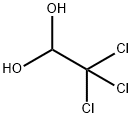
6335-41-7
22 suppliers
inquiry

39755-95-8
206 suppliers
$8.00/1g
Yield:39755-95-8 88%
Reaction Conditions:
with sulfuric acid in water at 70 - 80; for 1.25 h;
Steps:
2) 5-Methoxy-isatin
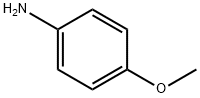
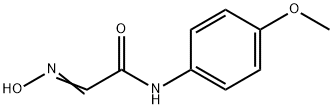
Concentrated sulfuric acid (176 mL) containing 5 mL of water was warmed to 70 0C and N-(2-hydroximino-acetyl)anisidine (25 g, 0.13 mol) was added portionwise over 1 h maintaining the temperature between 70-80 0C. After complete addition the mixture was refluxed at 80 0C for 15 minutes. Then mixture was poured into 500 g of ice and 800 mL of cold water. The black precipitate was collected by vacuum filtration, washed with cold water to pH 7 and dried on air to give 21.78 g (88 % yield) of 5-methoxy-isatin. mp (exp.) = 201-205 0C (mp [2] = 204-204.5 0C). Spectrum NMR 1 (CDCl3, δ, ppm, J, Hz): 3.74 (s, 3); 6.84 (d, 1, J =8.6); 7.06 (s, 1); 7.17 (dd, 1H, J =1.0, J =2.5); 10.86 (s, 1).
References:
Zaryanova, Ekaterina V.;Lozinskaya, Nataly A.;Beznos, Olga V.;Volkova, Maria S.;Chesnokova, Nataly B.;Zefirov, Nikolay S. [Bioorganic and Medicinal Chemistry Letters,2017,vol. 27,# 16,p. 3787 - 3793] Location in patent:supporting information
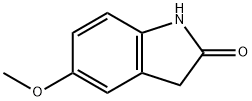
7699-18-5
127 suppliers
$8.00/100mg

39755-95-8
206 suppliers
$8.00/1g
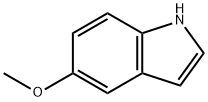
1006-94-6
479 suppliers
$10.00/5g

39755-95-8
206 suppliers
$8.00/1g
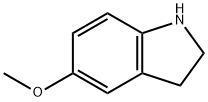
21857-45-4
120 suppliers
$15.00/100mg

39755-95-8
206 suppliers
$8.00/1g