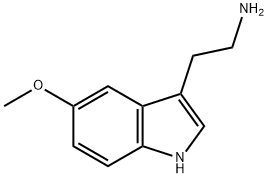
5-Methoxytryptamine synthesis
- Product Name:5-Methoxytryptamine
- CAS Number:608-07-1
- Molecular formula:C11H14N2O
- Molecular Weight:190.24

![Ethyl 3-[2-(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)ethyl]-5-methoxy-1H-indole-2-carboxylate](/CAS/20140525/GIF/CB12555002.gif)
55747-53-0
11 suppliers
inquiry

608-07-1
446 suppliers
$13.00/5g
Yield:608-07-1 99%
Reaction Conditions:
Stage #1: 2-carboxyethyl-3-(2-phthalimidoethyl)-5-methoxyindolewith lithium hydroxide monohydrate;sodium hydroxide at 88 - 90;
Stage #2: with hydrogenchloride in lithium hydroxide monohydrate at 10 - 110;Temperature;
Steps:
1.3; 9
Add 200 g of water and 40 g of sodium hydroxide to the reaction vessel, wait for the solution to dissolve at room temperature, add 248.5 g of compound II, warm to 88-90 ° C, and react for 5-6 hours; 10 ~ 20 ° C, slowly add 192.4g of concentrated hydrochloric acid (36wt%), after the addition is complete, raise the temperature to 107 ~ 110 ° C, and react for 1 ~ 2h; then reduce the reaction solution to room temperature, filter, and wash the filter cake with water until neutral It was dried at 55 ° C to obtain 119.3 g of pure compound III with a yield of 99.0%.
References:
CN110818610,2020,A Location in patent:Paragraph 0049; 0055; 0087-0089
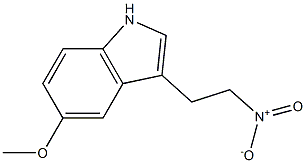
68935-49-9
1 suppliers
inquiry

608-07-1
446 suppliers
$13.00/5g
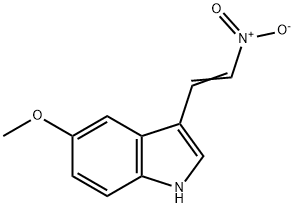
61675-19-2
26 suppliers
$34.00/25mg

608-07-1
446 suppliers
$13.00/5g

61675-19-2
26 suppliers
$34.00/25mg

608-07-1
446 suppliers
$13.00/5g
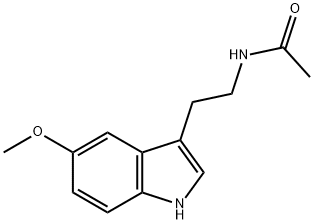
73-31-4
804 suppliers
$6.00/1g

608-07-1
446 suppliers
$13.00/5g