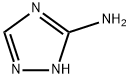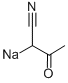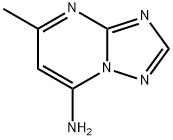
5-METHYL[1,2,4]TRIAZOLO[1,5-A]PYRIMIDIN-7-AMINE synthesis
- Product Name:5-METHYL[1,2,4]TRIAZOLO[1,5-A]PYRIMIDIN-7-AMINE
- CAS Number:33376-96-4
- Molecular formula:C6H7N5
- Molecular Weight:149.15
![7-CHLORO-5-METHYL-1,2,4-TRIAZOLE[1,5-A]PYRIMIDINE](/CAS/GIF/24415-66-5.gif)
24415-66-5
71 suppliers
$16.00/250mg
![5-METHYL[1,2,4]TRIAZOLO[1,5-A]PYRIMIDIN-7-AMINE](/CAS/GIF/33376-96-4.gif)
33376-96-4
29 suppliers
$45.00/50mg
Yield:33376-96-4 73%
Reaction Conditions:
with ammonium hydroxide for 1 h;
Steps:
4.2. Synthesis of 7-amino-5-methyl-[1,2,4]triazolo[1,5-a]pyrimidine (7-amtp·H2O)
General procedure: The triazolopyrimidinic derivative used as a ligand was prepared following the method described by Makisumi [64], in which 29 mmol (0.5 g) of 7-hydroxy-5-methyl-1,2,4-triazolo[1,5-a]pyrimidine (HmtpO) were added on a round flask with 10 mL of phosphoryl chloride and put in reflux for 90 min, while the mixture turned dark orange. After this time, solution is cooled up to room temperature and the mixture is basified with sodium hydrogen carbonate until there was no visible reaction. Then, the solution is extracted with dichloromethane and the chlorated intermediate is collected by using a rotoevaporator. Obtained product (7-chloro-5-methyl-1,2,4-triazolo[1,5-a]pyrimidine) was putted in a excess of commercial ammonia solution and magnetically stirred during an hour. After this time, yellow crystals of amino derivative (7-amtp) were obtained, some of them suitable for XRD measures. Yield: 73%, based on HmtpO. Anal. Calcd.C6H9N5O: C, 43.11; H, 5.43; N, 41.89. Found: C, 43.22; H, 5.39; N,42.01. IR: 1483 cm-1 (N-H(flex)), 1573 cm-1 (py), 1660 cm-1 (tp),3096 cm-1 (O-H) and 3300 cm-1 (N-H(tens)).
References:
Esteban-Parra, Ginés M.;Sebastián, Eider San;Cepeda, Javier;Sánchez-González, Cristina;Rivas-García, Lorenzo;Llopis, Juan;Aranda, Pilar;Sánchez-Moreno, Manuel;Quirós, Miguel;Rodríguez-Diéguez, Antonio [Journal of Inorganic Biochemistry,2020,vol. 212,art. no. 111235]
![4-methyl-1,5,7,9-tetrazabicyclo[4.3.0]nona-3,5,7-trien-2-one](/CAS/20180531/GIF/35523-67-2.gif)
35523-67-2
9 suppliers
inquiry
![5-METHYL[1,2,4]TRIAZOLO[1,5-A]PYRIMIDIN-7-AMINE](/CAS/GIF/33376-96-4.gif)
33376-96-4
29 suppliers
$45.00/50mg
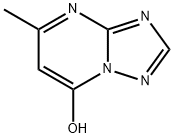
2503-56-2
165 suppliers
$9.00/5g
![5-METHYL[1,2,4]TRIAZOLO[1,5-A]PYRIMIDIN-7-AMINE](/CAS/GIF/33376-96-4.gif)
33376-96-4
29 suppliers
$45.00/50mg