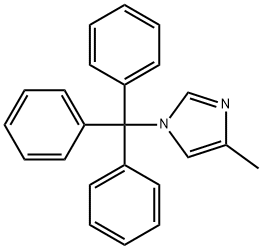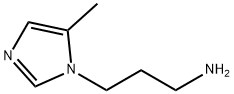
5-methyl-1H-Imidazole-1-propanamine synthesis
- Product Name:5-methyl-1H-Imidazole-1-propanamine
- CAS Number:279236-77-0
- Molecular formula:C7H13N3
- Molecular Weight:139.2
Yield:279236-77-0 144 mg
Reaction Conditions:
Stage #1: 2-(3-bromopropyl)isoindole-1,3-dione;4-methyl-1-trityl-1H-imidazole in acetonitrile; for 3 h;Reflux;Gabriel Amine Synthesis;
Stage #2: with trifluoroacetic acid in methanol; for 3 h;Reflux;Gabriel Amine Synthesis;
Stage #3: with hydrazine hydrate in ethanol at 20; for 2 h;Gabriel Amine Synthesis;
Steps:
4.1.7.23. 2-(3-(5-Methyl-1H-imidazol-1-yl)propyl)isoindoline-1,3-dione (39). 4.1.7.24. 3-(5-Methyl-1H-imidazol-1-yl)propan-1-amine(40).
Compound 37 (3 g, 9.26 mmol) was suspended in 30 mL acetonitrile and 2-(3-bromopropyl)phthalimide (1.48 g, 9.26 mmol) was added. The mixture was kept under reflux overnight and concentrated to give 38. Crude mixture 38 was dissolved in a stirred solution containing methanol (20 mL) and trifluoroacetic acid (3 mL). The mixture was kept under reflux overnight. The solvent was removed, and the remaining oil was purified by flash chromatography using silica gel and a MC/MeOH gradient. Yield: 1.52 g (61%). 4.1.7.24. 3-(5-Methyl-1H-imidazol-1-yl)propan-1-amine(40). Compound 39 (300 mg, 1.11 mmol) was dissolved in ethanol (2 mL) and hydrazine monohydrate (0.27 ml, 5.55 mmol) was added dropwise. The mixture was stirred at room temperature for 2 h. The formed precipitate was filtered off and washed with EtOH. The filtrate was collected and concentrated by rotary evaporation. The yellow oil product 3-(5-methyl-1H-imidazol-1-yl)propan-1-amine 40 (144 mg, 93%) was carried on to the next step without further purification. 1H NMR (300 MHz, CD3OD): d 7.56 (d, J= 0.9 Hz, 1H), 6.67 (s, 1H), 4.01 (t, J= 7.14 Hz, 2H), 2.65 (t, J= 7.14 Hz, 2H), 2.22 (d, J= 0.93 Hz, 3H), 1.92 (quintet, J= 7.32 Hz, 2H). MS (FAB) m/z: 140 [M+H]+.
References:
Lee, Jeewoo;Tran, Phuong-Thao;Hoang, Van-Hai;Thorat, Shivaji A.;Kim, Sung Eun;Ann, Jihyae;Chang, Yu Jin;Nam, Dong Woo;Song, Hyundong;Mook-Jung, Inhee;Lee, Jiyoun [Bioorganic and Medicinal Chemistry,2013,vol. 21,# 13,p. 3821 - 3830]
822-36-6
481 suppliers
$9.00/1g
5460-29-7
272 suppliers
$7.00/5g

279236-77-0
10 suppliers
inquiry

78881-20-6
1 suppliers
inquiry