
5-methyl-1H-Indole-2-methanol synthesis
- Product Name:5-methyl-1H-Indole-2-methanol
- CAS Number:55795-87-4
- Molecular formula:C10H11NO
- Molecular Weight:161.2
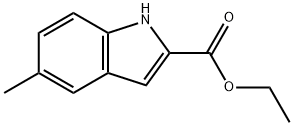
16382-15-3
140 suppliers
$18.00/1g

55795-87-4
22 suppliers
inquiry
Yield:55795-87-4 98%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran at 0 - 20;Inert atmosphere;
Steps:
2.1. Typical procedure for the preparation of (1H-indol-2-yl)methanols: synthesis of the (1H-indol-2-yl)methanol
General procedure: A flame dried three-necked round bottom flask, equipped with a magnetic stirring bar, was charged with ethyl 1H-indole-2-carboxylate (1.5 g, 7.93 mmol, 1.0 equiv.) dissolved in anhydrous THF (20 mL) under argon. Then, a solution of LiAlH4 in THF (2 M, 4.4 mL, 8.72 mmol, 1.1 equiv.) was added dropwise at 0°C and the mixture was stirred for 1 hour. After this time, the mixture was allowed to warm to room temperature and stirred until the disappearance of the starting material, monitoring by TLC (mobile phase for TLC: n-hexane-EtOAc, 80:20). The reaction was cooled down to 0°C and quenched with an 80 percent aqueous MeOH solution. The mixture was extracted with CH2Cl2, washed with a solution of KHSO4 (10% w/w) and with brine. The organic layer was dried over MgSO4, filtered, and concentrated under reduced pressure. The resulting crude product was used in the next step without further purification (1.108 g, 95% yield).
References:
Arcadi, Antonio;Calcaterra, Andrea;Chiarini, Marco;Fabrizi, Giancarlo;Fochetti, Andrea;Goggiamani, Antonella;Iazzetti, Antonia;Marrone, Federico;Marsicano, Vincenzo;Serraiocco, Andrea [Synthesis,2021,vol. 54,# 3,p. 741 - 753] Location in patent:supporting information
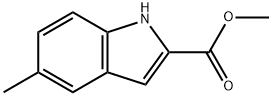
102870-03-1
34 suppliers
inquiry

55795-87-4
22 suppliers
inquiry
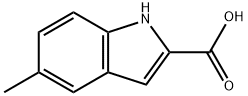
10241-97-1
143 suppliers
$8.00/100mg

55795-87-4
22 suppliers
inquiry