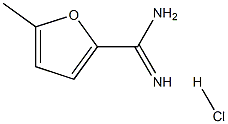
5-methyl-2-furancarboximidamide hydrochloride synthesis
- Product Name:5-methyl-2-furancarboximidamide hydrochloride
- CAS Number:856173-27-8
- Molecular formula:C6H9ClN2O
- Molecular Weight:160.6015
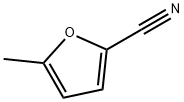
13714-86-8
42 suppliers
$60.00/10mg

856173-27-8
8 suppliers
inquiry
Yield:856173-27-8 97.6%
Reaction Conditions:
Stage #1: 5-methyl-furan-2-carbonitrilewith sodium methylate in methanol at 30; for 3 h;
Stage #2: with ammonium chloride in methanol at 30; for 37 h;
Steps:
1.2 Step 2: Preparation of 5-methylfuran-2-carboxamidine hydrochloride
Step 2:
Preparation of 5-methylfuran-2-carboxamidine hydrochloride
To a solution of NaOMe (1.55 g, 28.77 mmol, 0.1 eq.) in MeOH (350 mL) was added 5-methylfuran-2-carbonitrile (32 g, 286.81 mmol, 1 eq.).
The mixture was stirred at 30° C. for 3 hours To the resulting solution was slowly added NH4Cl (16.88 g, 315.49 mmol, 1.1 eq.) and the mixture was stirred at 30° C. for 37 h.
The resulting suspension was filtered and the solvent removed under reduced pressure.
The solid obtained was washed with methyl tert-butyl ether (200 mL) to give the title compound, 5-methylfuran-2-carboxamidine hydrochloride (45 g, 97.6% yield) was obtained as a light yellow solid. LCMS m/z 124.9 [M+H]+; 1HNMR (d6-DMSO 400 MHz) δ 8.73 (br s, 4H), 7.86 (d, J=3.2 Hz, 1H), 6.52-6.41 (m, 1H), 2.38 (s, 3H).
References:
US2021/292332,2021,A1 Location in patent:Paragraph 0221; 0224-0225
620-02-0
480 suppliers
$14.14/5gm:

856173-27-8
8 suppliers
inquiry