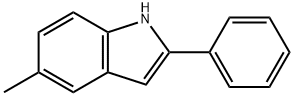
5-METHYL-2-PHENYLINDOLE synthesis
- Product Name:5-METHYL-2-PHENYLINDOLE
- CAS Number:13228-36-9
- Molecular formula:C15H13N
- Molecular Weight:207.27
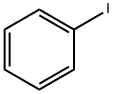
591-50-4
478 suppliers
$10.00/1g
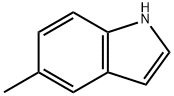
614-96-0
311 suppliers
$19.00/1g

13228-36-9
33 suppliers
$48.00/250 mg
Yield: 89%
Reaction Conditions:
with norborn-2-ene;dichloro bis(acetonitrile) palladium(II);potassium carbonate in N,N-dimethyl acetamide;water at 70;regioselective reaction;
Steps:
2. General procedure for 2-arylation of free (N-H) indoles
General procedure: A high pressure tube equipped with a magnetic stirring bar was charged with indole substrate (0.2mmol, 1 equiv.), norbornene (0.4mmol, 2 equiv.), the base (0.4mmol, 2 equiv. K2CO3 or 2 equiv. KHCO3, as indicated),, and PdCl2(MeCN)2 (0.02mmol, 10 mol %). A solution of water (0.5 M) in DMA (1mL) was added via syringe, and then the aryl iodide (0.4mmol, 2 equiv.) was added via syringe. The reaction mixture was then placed in a preheated oil bath at 70 °C (or 90 °C, as indicated). Vigorous stirring was applied. The reaction was monitored by TLC. Upon completion, the reaction mixture was cooled to room temperature, diluted with EtOAc, and filtered. The filtrate was extracted with H2O (3 times) and brine, the organic phase was concentrated in vacuum to remove the solvent. The residue was directly submitted to flash column chromatography to afford the 2-arylindole product. The spectral data of the products were in accordance with those reported in the literature1.
References:
Gao, Yadong;Zhu, Wangying;Yin, Long;Dong, Bo;Fu, Jingjing;Ye, Zhiwen;Xue, Fengtian;Jiang, Chao [Tetrahedron Letters,2017,vol. 58,# 23,p. 2213 - 2216] Location in patent:supporting information