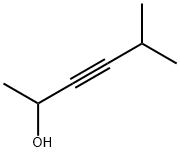
5-Methyl-3-hexyn-2-ol synthesis
- Product Name:5-Methyl-3-hexyn-2-ol
- CAS Number:23293-50-7
- Molecular formula:C7H12O
- Molecular Weight:112.17
Yield:23293-50-7 96%
Reaction Conditions:
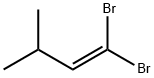
Stage #1: 1,1-dibromo-3-methyl-1-butenewith n-butyllithium in tetrahydrofuran;hexane at -78; for 0.5 h;
Stage #2: acetaldehyde in tetrahydrofuran;hexane at -78;
Steps:
5-Methylhex-3-yn-2-one (15)
To a solution of the above dibromide (1.86 g, 8.16 mmol) in THF (20 mL) was addedn-BuLi (1.63 M in hexane, 10.0 mL, 16.3 mmol) dropwise at -78 oC. After 30 min of stirringat -78 oC, acetaldehyde (0.68 mL, 12.2 mmol) was added dropwise. The solution was allowedto warm to 0 oC over 1 h and diluted with saturated NH4Cl. The resulting mixture wasextracted with CH2Cl2 three times. The combined extracts were dried over MgSO4 andconcentrated to give a residue, which was purified by chromatography on silica gel(CH2Cl2/petroleum ether) to afford the racemic alcohol 16 (877 mg, 96%).
References:
Kobayashi, Yuichi;Saeki, Ryohei;Nanba, Yutaro;Suganuma, Yuta;Morita, Masao;Nishimura, Keita [Synlett,2017,vol. 28,# 19,p. 2655 - 2659] Location in patent:supporting information