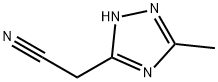
(5-METHYL-4H-1,2,4-TRIAZOL-3-YL)ACETONITRILE synthesis
- Product Name:(5-METHYL-4H-1,2,4-TRIAZOL-3-YL)ACETONITRILE
- CAS Number:86999-26-0
- Molecular formula:C5H6N4
- Molecular Weight:122.13
Yield: 74%
Reaction Conditions:
with sodium hydroxide in methanol for 2 h;Heating / reflux;
Steps:
77 5-Cyanomethyl-3-methyl-1H-[1,2,4]triazole (I-77)
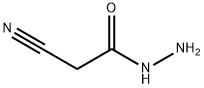
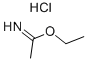
A methanol (60 ml) solution of 3.39 g (95%, 34.2 mmol) of sodium hydroxide was mixed with 4.10 g (33.2 mmol) of ethyl acetimidate hydrochloride (I-55) and 3.39 g (34.2 mmol) of cyanoacetohydrazide and heated under reflux for 2 hours. After cooling, the reaction solution was concentrated under a reduced pressure, the thus obtained residue was mixed with ethanol, the insoluble material was removed by filtration, and the solvent of the filtrate was evaporated. The thus obtained residue was applied to a silica gel column chromatography, and 3.01 g (74%) of the title compound was obtained as a colorless solid from a chloroform-methanol (30:1 v/v) eluate. MS (FAB)m/z: 123 (M+1)+. 1H-NMR (DMSO-d6)δ: 2.32 (3H, s), 4.03 (2H, s).
References:
DAIICHI PHARMACEUTICAL CO., LTD. EP1717238, 2006, A1 Location in patent:Page/Page column 62