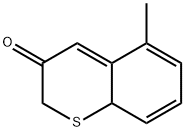
5-Methylbenzo[b]thiophen-3(2H)-one synthesis
- Product Name:5-Methylbenzo[b]thiophen-3(2H)-one
- CAS Number:56639-88-4
- Molecular formula:C9H8OS
- Molecular Weight:164.22
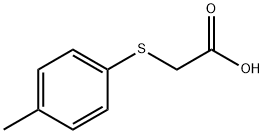
3996-29-0
78 suppliers
$30.00/5g
![5-Methylbenzo[b]thiophen-3(2H)-one](/CAS/GIF/56639-88-4.gif)
56639-88-4
15 suppliers
inquiry
Yield:56639-88-4 70%
Reaction Conditions:
Stage #1: 2-[(4-methylphenyl)thio]acetic acidwith oxalyl dichloride;N,N-dimethyl-formamide in dichloromethane at 25; for 1 h;
Stage #2: with aluminum (III) chloride in dichloromethane at 0 - 25; for 2 h;
Steps:
62.2 Step 2: 5-methylbenzo[b]thiophen-3(2H)-one (393-3)
To a stirred solution of Compound 393-2 (600 mg, 3.3 mmol) in DCM (15 mL) was added (COCl)2(627mg, 4.9 mmol) at 0°C, followed by a drop of DMF (24 mg, 329 μmol). The mixture was stirred at 25°C for 1 hr. The mixture was concentrated in vacuum to give a residue, which was diluted with DCM (15 mL) and was added AlCl3 (702 mg, 5.3 mmol) at 0°C. The resulting mixture was stirred at 25°C for 2 hrs. LCMS showed the reaction was completed and one main peak with desired mass was detected. The reaction mixture was poured into H2O (20 mL) at 0°C, and then extracted with DCM (10 mL x 5). The combined organic layers were dried over Na2SO4, filtered and concentrated under reduced pressure to give a residue, which was purified by silica gel chromatography (Ethyl acetate / Petroleum) to give Compound 393-3 (380 mg, 2.3 mmol, 70% yield) as a pink solid. M+H+= 165.0 (LCMS),1H NMR (400MHz, CHLOROFORM-d) d = 7.60 (s, 1H), 7.42 - 7.36 (m, 1H), 7.35 - 7.30 (m, 1H), 3.80 (s, 2H), 2.38 (s, 3H).
References:
WO2021/3295,2021,A1 Location in patent:Paragraph 0621
![Acetyl chloride, 2-[(4-methylphenyl)thio]-](/CAS/20210305/GIF/30893-63-1.gif)
30893-63-1
0 suppliers
inquiry
![5-Methylbenzo[b]thiophen-3(2H)-one](/CAS/GIF/56639-88-4.gif)
56639-88-4
15 suppliers
inquiry
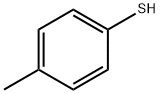
106-45-6
208 suppliers
$10.00/1g
![5-Methylbenzo[b]thiophen-3(2H)-one](/CAS/GIF/56639-88-4.gif)
56639-88-4
15 suppliers
inquiry