
5-METHYLISOINDOLINE synthesis
- Product Name:5-METHYLISOINDOLINE
- CAS Number:93282-20-3
- Molecular formula:C9H11N
- Molecular Weight:133.19
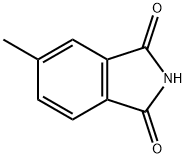
40314-06-5
59 suppliers
$26.00/1g

93282-20-3
32 suppliers
inquiry
Yield:93282-20-3 27%
Reaction Conditions:
Stage #1: 5-methyl-1H-isoindole-1,3-(2H)-dionewith borane-THF in tetrahydrofuran at 20 - 60;
Stage #2: with hydrogenchloride in tetrahydrofuran;methanol;water at 0; for 1 h;Heating / reflux;
Steps:
71
The 4-methylphthalimide (1.8 g) obtained above was suspended in tetrahydrofuran (3 ml), then 1 N borane tetrahydrofuran complex (30 ml) was added thereto at room temperature and stirred overnight at 60°C. The mixture was cooled to 0°C, then methanol (2.8 ml) and 6 N hydrochloric acid (3.2 ml) were added thereto, and the mixture was refluxed for 1 hour. The reaction mixture was cooled to 0°C, then 6 N sodium hydroxide solution was added thereto, and the reaction solution was extracted with ethyl acetate and then the extract was dried over sodium sulfate anhydrous. The resulting product was concentratedunderreducedpressure, andtheresiduewaspurified by column chromatography (eluding solvent; dichloromethane → dichloromethane: methanol 10: 1 → 5: 1) to give the title compound (400 mg, Y.: 27%). 1H NMR; (CDCl3) δ (ppm): 2.3 (3H, s), 2.7 (1H, brs), 7.0 (1H, d), 7.1 (1H, s), 7.2 (1H, d). ESI/MS (m/z): 134 (M+H)+.
References:
EP1595866,2005,A1 Location in patent:Page/Page column 27
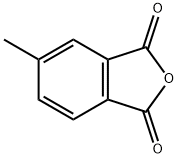
19438-61-0
197 suppliers
$8.00/5g

93282-20-3
32 suppliers
inquiry