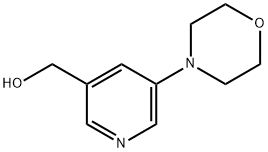
(5-Morpholinopyridin-3-yl)Methanol synthesis
- Product Name:(5-Morpholinopyridin-3-yl)Methanol
- CAS Number:888070-06-2
- Molecular formula:C10H14N2O2
- Molecular Weight:194.23

500865-54-3
6 suppliers
inquiry

888070-06-2
11 suppliers
inquiry
Yield:888070-06-2 80%
Reaction Conditions:
Stage #1: methyl 5-morpholin-4-yl-nicotinatewith lithium aluminium tetrahydride in tetrahydrofuran at -40; for 2 h;
Stage #2: with sodium hydroxide;water in tetrahydrofuran at -40 - 20; for 0.5 h;
Steps:
VI; 13.2
To a suspension of LAH (1 g, 0.027 mol) in dry THF (70 mL) was added methyl 5-morpholin-4-ylnicotinate (4 g, 0.018 mol) in dry THF (10 mL) at -40° C. under stirring. The reaction mixture was stirred at this temperature for 2 h and then quenched with 6 mL of 10% aqueous NaOH solution at -40° C. The reaction mixture was allowed to stir at RT for 30 min, filtered through celite, washed with THF and concentrated to afford 2.8 g, (80%) of the title compound as a liquid. TLC-Chloroform/Methanol (8/2): Rf=0.55. 1H NMR (DMSO-d6) δ 8.17 (d, J=2.6 Hz, 1H), 7.97 (s, 1H), 7.23 (s, 1H), 5.24 (t, J=5.6 Hz, 1H), 4.47 (d, J=5.6 Hz, 2H), 3.75-3.72 (m, 4H), 3.15-3.12 (m, 4H).
References:
US2008/51397,2008,A1 Location in patent:Page/Page column 10; 26

29681-44-5
297 suppliers
$6.00/5g

888070-06-2
11 suppliers
inquiry