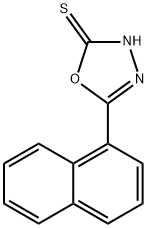
5-(naphthalen-1-yl)-1,3,4-oxadiazole-2-thiol synthesis
- Product Name:5-(naphthalen-1-yl)-1,3,4-oxadiazole-2-thiol
- CAS Number:116082-89-4
- Molecular formula:C12H8N2OS
- Molecular Weight:228.27

75-15-0
242 suppliers
$40.00/100g

43038-45-5
103 suppliers
$45.00/1g

116082-89-4
7 suppliers
inquiry
Yield:116082-89-4 71%
Reaction Conditions:
Stage #1: 1-naphthohydrazidewith potassium hydroxide in methanol at 20; for 0.5 h;
Stage #2: carbon disulfide in methanol at 70; for 24 h;Reflux;
Steps:
Synthesis of 5-(p-tolyl)-1,3,4-oxadiazole-2-thiol (3a), 2-(5-mercapto-1,3,4-oxadiazol-2-yl)phenol (3b), 5-(naphthalen-1-yl)-1,3,4-oxadiazole-2-thiol (3c).
General procedure: Intermediate 2a (180.23 mg, 1 mmol), KOH (1.1 mmol, 56.1 mg) and 12 mL of MeOH were added to a two-necked flask, and the reaction was carried out at room temperature for 30 minutes. After the addition of CS2 (100 mmol, 7.6 g), the mixture was stirred at 70 °C for 20 h. Then 1 mmol CS2 (76.0 mg) was added and it refluxed for 4 h. When the reaction completed, 100 mL water was added, and the remained CS2 and MeOH were removed under reduced pressure. After the mixture was suspended in water, the pH was adjusted to 3-4 by adding concentrated HCl. The residue was filtered and washed three times with water to give a white solid 3a (130.72 mg, yield 68%).1H NMR (400 MHz, DMSO-d6) δ(ppm) 14.70(s, 1H), 7.77-7.75 (d, J=8.0Hz, 2H), 7.40-7.38 (d, J=8.0Hz, 2H), 2.38 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ(ppm) 177.26, 160.61, 142.52, 129.96, 126.00, 119.69, 21.14. HR-MS (TOF): m/z Calcd. For C9H9N2OS (M + H+): 193.0430; Found:193.0429.
References:
Zhao, Yu Qiang;Zhou, Jie;He, Renze;Wang, Guang Ke;Miao, Lan Xi;Xie, Xiao Guang;Zhou, Ying [Tetrahedron Letters,2020,vol. 61,# 52,art. no. 152668] Location in patent:supporting information

86-55-5
366 suppliers
$5.00/5g

116082-89-4
7 suppliers
inquiry
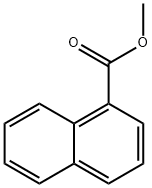
2459-24-7
87 suppliers
$33.00/5g

116082-89-4
7 suppliers
inquiry
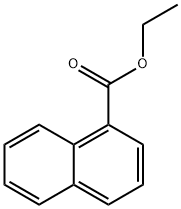
3007-97-4
59 suppliers
$23.00/1g

116082-89-4
7 suppliers
inquiry

43038-45-5
103 suppliers
$45.00/1g

116082-89-4
7 suppliers
inquiry